Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-DAD Method Development
2.2. Identification of Phenolic Compounds in C. subternata
2.2.1. MS/MS Fragmentation of C. subternata Phenolics
2.2.2. Benzophenones
2.2.3. Dihydrochalcones
2.2.4. Flavone and Flavanone C-glycosides
2.2.5. Flavone and Flavanone O-Glucosides
2.2.6. Additional Unidentified Phenolic Compounds
2.3. HPLC-DAD Method Validation
2.4. Differences in Phenolic Composition and TAC of Aqueous Extracts of C. subternata Leaves and Stems
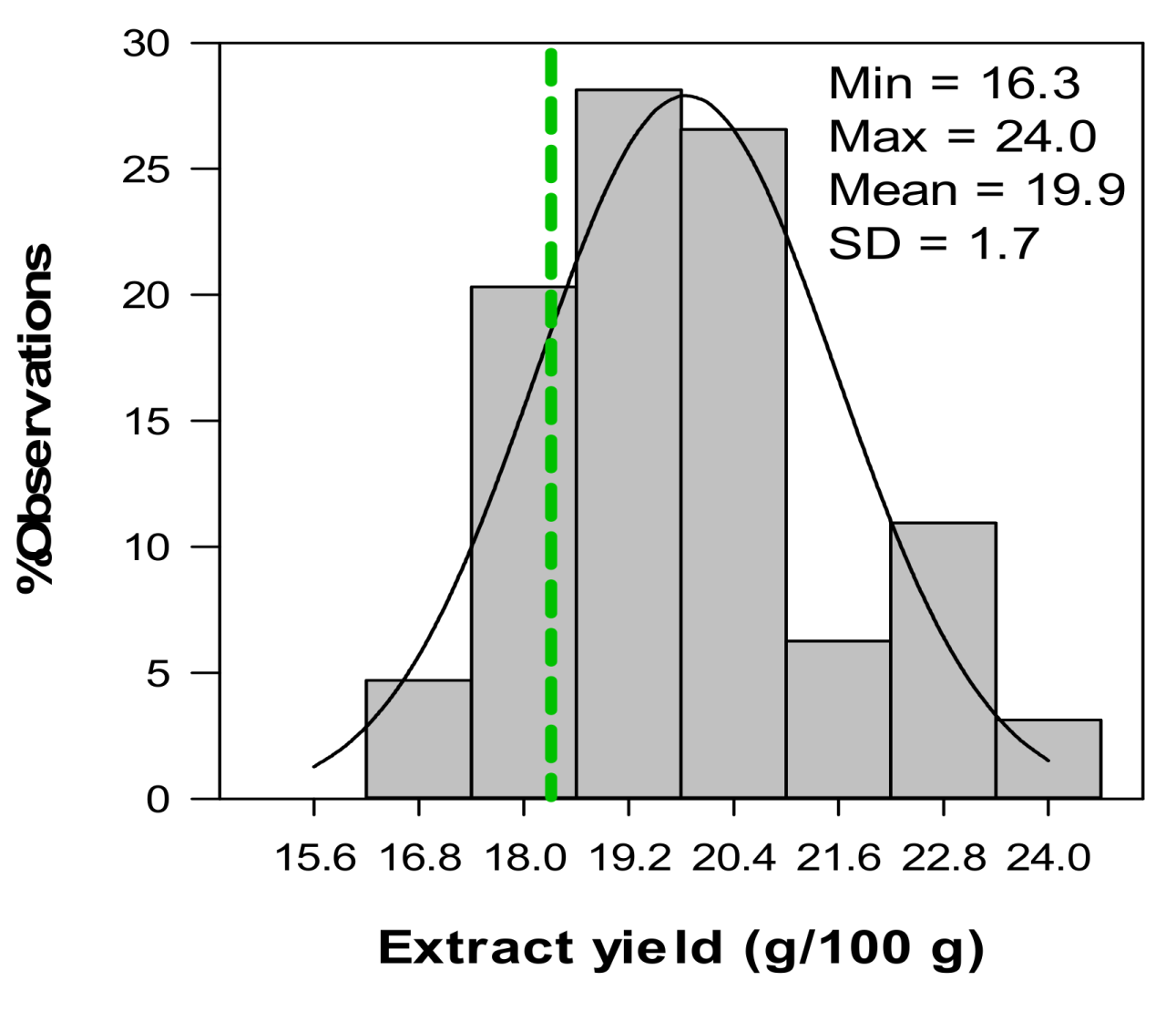
2.5. Variation in Phenolic Composition and TAC of Aqueous Extracts from C. subternata Seedlings from Combined Leaf and Stem Material
3. Experimental
3.1. Chemicals
3.2. Sourcing of Plant Material
3.3. Preparation of Aqueous Extracts
3.4. HPLC-DAD Method Development and Validation
3.4.1. Method Development
3.4.2. Quantification of Individual Phenolic Compounds using HPLC-DAD
3.4.3. Identification of Phenolic Compounds using LC-DAD-MS and -MS/MS Detection
3.4.4. Method Validation
3.5. Determination of Total Phenol Content and Total Antioxidant Capacity (TAC)
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Roberts, C.; Sindhu, K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Ou, B.; Bell, D.; Zhao, Q.; Wei, H. Antioxidant science: From antioxidants to ‘antiagents’. Nutraceuticals World 2011, 14, 46–49. [Google Scholar]
- Joubert, E.; Joubert, M.E.; Bester, C.; de Beer, D.; de Lange, J.H. Honeybush (Cyclopia spp.): From local cottage industry to global markets—The catalytic and supporting role of research. S. Afr. J. Bot. 2011, 77, 887–907. [Google Scholar] [CrossRef]
- de Beer, D.; Jerz, G.; Joubert, E.; Wray, V.; Winterhalter, P. Isolation of isomangiferin from honeybush (Cyclopia subternata) using high-speed counter-current chromatography and high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 4282–4289. [Google Scholar] [CrossRef] [PubMed]
- Kamara, B.I.; Brand, D.J.; Brandt, E.V.; Joubert, E. Phenolic metabolites from honeybush tea (Cyclopia subternata). J. Agric. Food Chem. 2004, 52, 5391–5395. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Luczkiewicz, M.; Sowinski, P.; Glod, D.; Gorynski, K.; Bucinski, A. Isolation and structure elucidation of phenolic compounds from Cyclopia subternata Vogel (honeybush) intact plant and in vitro cultures. Food Chem. 2012, 133, 1373–1382. [Google Scholar] [CrossRef]
- Joubert, E.; Gelderblom, W.C.A.; de Beer, D. Phenolic contribution of South African herbal teas to a healthy diet. Nat. Prod. Commun. 2009, 4, 701–718. [Google Scholar] [PubMed]
- Mose Larsen, P.; Fey, S.J.; Louw, J.; Joubert, L. An anti-diabetic extract of honeybush. World Intellectual Property Organization. WO. 2008/110552A2, 2008. [Google Scholar]
- Sánchez, G.M.; Re, L.; Giuliani, A.; Núñez-Sellés, A.J.; Davison, G.P.; León-Fernández, O.S. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol. Res. 2000, 42, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.M.; Álvarez, E.; Arranz, J.A.; Siso, I.G.; Orallo, F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-α and transforming growth factor-β genes. Biochem. Pharmacol. 2003, 65, 1361–1371. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Mitra, A.; Manjunatha, M. Studies on the anti-diabetic and hypolipidemic potentials of mangiferin (xanthone glucoside) in streptozotocin-induced Type 1 and Type 2 diabetic model rats. Int. J. Adv. Pharm. Sci. 2010, 1, 75–85. [Google Scholar]
- Pardo Andreu, G.L.; Maurmann, N.; Reolon, G.K.; de Farias, C.B.; Schwartsmann, G.; Delgado, R.; Roesler, R. Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur. J. Pharmacol. 2010, 635, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.T.; Yap, S.-A.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Standardised Mangifera indica extract is an ideal antioxidant. Food Chem. 2009, 113, 1154–1159. [Google Scholar] [CrossRef]
- Núñez Sellés, A.J.; Vélez Castro, H.T.; Agüero-Agüero, J.; González-González, J.; Naddeo, F.; de Simone, F.; Rastrelli, L. Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef] [PubMed]
- de Beer, D.; Joubert, E. Development of HPLC method for Cyclopia subternata phenolic compound analysis and application to other Cyclopia spp. J. Food Comp. Anal. 2010, 23, 289–297. [Google Scholar] [CrossRef]
- Joubert, E.; Richards, E.S.; van der Merwe, J.D.; de Beer, D.; Manley, M.; Gelderblom, W.C.A. Effect of species variation and processing on phenolic composition and in vitro antioxidant activity of aqueous extracts of Cyclopia spp. (honeybush tea). J. Agric. Food Chem. 2008, 56, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.-K.; Jeong, K.-S.; Choi, M.-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Minato, K.-I.; Miyake, Y.; Fukumoto, S.; Yamamoto, K.; Kato, Y.; Shimomura, Y.; Osawa, T. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci. 2003, 72, 1609–1616. [Google Scholar] [CrossRef]
- Miyake, Y.; Suzuki, E.; Ohya, S.; Fukumoto, S.; Hiramitsu, M.; Sakaida, K.; Osawa, T.; Furuichi, Y. Lipid-lowering effect of eriocitrin, the main flavonoid in lemon fruit, in rats on a high-fat and high-cholesterol diet. J. Food Sci. 2006, 71, S633–S637. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K.; Osawa, T. Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon BURM. f.) and its antioxidative activity. Food Sci. Technol. Int. Tokyo 1997, 3, 84–89. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Jung, H.A.; Islam, M.D.N.; Kwon, Y.S.; Jin, S.E.; Son, Y.K.; Park, J.J.; Sohn, H.S.; Choi, J.S. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem. Toxicol. 2011, 49, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.-Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Botha, M.; Maicu, C.; de Beer, D.; Manley, M. Rapid screening methods for estimation of mangiferin and xanthone contents of Cyclopia subternata plant material. S. Afr. J. Bot. 2012, 82, 113–122. [Google Scholar] [CrossRef]
- Abad-García, B.; Berrueta, L.A.; Garmón-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef] [PubMed]
- Beelders, T.; Sigge, G.O.; Joubert, E.; de Beer, D.; de Villiers, A. Kinetic optimisation of the reversed phase liquid chromatographic separation of rooibos tea (Aspalathus linearis) phenolics on conventional high performance liquid chromatographic instrumentation. J. Chromatogr. A 2012, 1219, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Beelders, T.; Sigge, G.O.; Joubert, E.; de Beer, D.; de Villiers, A. Erratum to “Kinetic optimisation of the reversed phase liquid chromatographic separation of rooibos tea (Aspalathus linearis) phenolics on conventional high performance liquid chromatographic instrumentation”. [J. Chromatogr. A 1219 (2012) 128–139]. J. Chromatogr. A 2012, 1241, 128. [Google Scholar] [CrossRef]
- Beelders, T.; Kalili, K.M.; Joubert, E.; de Beer, D.; de Villiers, A.J. Comprehensive two-dimensional liquid chromatographic analysis of rooibos (Aspalathus linearis) phenolics. J. Sep. Sci. 2012, 35, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Debón, R.; Rull, A.; Rodríguez-Sanabria, F.; Iswaldi, I.; Herranz-López, M.; Aragonès, G.; Camps, J.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V.; et al. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine 2011, 18, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Kamara, B.I.; Brandt, E.V.; Ferreira, D.; Joubert, E. Polyphenols from honeybush tea (Cyclopia intermedia). J. Agric. Food Chem. 2003, 51, 3874–3879. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, S.; Yan, L.; Tai, J.; Xiao, Q.; Zou, K.; Zhou, Y.; Wu, J. Separation of flavanone enantiomers and flavanone glucoside diastereomers from Balanophora involucrata Hook. f. by capillary electrophoresis and reversed-phase high-performance liquid chromatography on a C18 column. J. Chromatogr. A 2008, 1185, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Beelders, T.; de Beer, D.; Malherbe, C.J.; de Villiers, A.J.; Sigge, G.O. Variation in phenolic content and antioxidant activity of fermented rooibos herbal tea infusions: Role of production season and quality grade. J. Agric. Food Chem. 2012, 60, 9171–9179. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; de Beer, D. Phenolic content and antioxidant activity of rooibos food ingredient extracts. J. Food Comp. Anal. 2012, 27, 45–51. [Google Scholar] [CrossRef]
- Bell, D.; Ou, B. The branding of ORAC: Where will it lead? Nutraceuticals World 2007, 10, 72–74. [Google Scholar]
- Joubert, E.; de Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. South Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Volz, R.K.; McGhie, T.K. Genetic variability in apple fruit polyphenol composition in Malus domestica and Malus sieversii germplasm grown in New Zealand. J. Agric. Food Chem. 2011, 59, 11509–11521. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.; Joubert, E.; de Beer, D.; Malherbe, C.J.; Witthuhn, R.C. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurisation of plant material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Peak a | Mode | tR (min) | Accurate massb | λmax (nm) | Error (ppm) | Proposed molecular formula | Fragments | Phenolic compound |
|---|---|---|---|---|---|---|---|---|
| 1 | + | 3.10 | 571.1664 | 290 | 0.2 | C25H31O15 | 373, 355, 337, 325, 313, 289, 271, 259, 231, 219, 195 *, 177, 165 | Iriflophenone-di-O,C-hexoside |
| − | 569.1488 | −2.3 | C25H29O15 | 479, 449, 317, 287 * | ||||
| 4 | + | 6.32 | 613.1780 | 285 | 1.8 | C27H33O16 | 475, 409, 339, 327, 303, 285, 261 *, 219 | (S)-Eriodictyol-di-C-hexoside |
| − | 611.1621 | 1.0 | C27H31O16 | 491, 431, 401, 371 * | ||||
| 5 | + | 6.50 | 613.1780 | 285 | 1.8 | C27H33O16 | 475, 409, 339, 327, 303, 285, 261 *, 219 | (R)-Eriodictyol-di-C-hexoside |
| − | 611.1621 | 1.0 | C27H31O16 | 491, 431, 401, 371 * | ||||
| 6 | + | 6.69 | 409.1136 | 294 | 0.2 | C19H21O10 | 391, 289, 231, 195 *, 177, 165, 121 | Iriflophenone-3-C-β-glucoside |
| − | 407.0981 | 1.0 | C19H19O10 | 317, 287 *, 257, 245, 215, 201, 193, 165, 125 | ||||
| 9 | + | 8.95 | 423.0920 | 234, 257, 317, 366 | −1.7 | C19H19O11 | 351, 339, 327, 303, 299, 285, 273 *, 257 | Mangiferin |
| − | 421.077 | −0.2 | C19H17O11 | 331, 301 *, 271, 259 | ||||
| 10 | + | 9.30 | 423.0922 | 234, 255, 316, 366 | −1.2 | C19H19O11 | 405, 357, 341, 327, 303 *, 299, 287, 285, 273, 261 | Isomangiferin |
| − | 421.0769 | −0.5 | C19H17O11 | 331, 301 *, 273, 271, 259 | ||||
| 11 | + | 9.40 | 595.1671 | 235, 270, 331 | 1.3 | C27H31O15 | 505, 457, 427, 421, 409, 391, 379, 355, 337, 325 *, 307, 295 | Apigenin-6,8-di-C-glucoside [Vicenin-2] |
| − | 593.1499 | −1.2 | C27H29O15 | 503, 473 *, 383, 353 | ||||
| 12 | + | 10.04 | 451.1229 | 281 | −2.4 | C21H23O11 | 289, 163, 153 * | Eriodictyol-O-glucoside |
| − | 449.1069 | −3.3 | C21H21O11 | 287, 151 *, 135 | ||||
| 13 | + | 10.63 | 451.1232 | 281 | −1.8 | C21H23O11 | 289 *, 163, 153 | Eriodictyol-O-glucoside |
| − | 449.1080 | −0.9 | C21H21O11 | 287, 151 *, 135, 107 | ||||
| 14 | + | 11.54 | 615.1927 | 283 | 0.3 | C27H35O16 | 525, 495, 477, 465, 447, 435, 423, 411, 399, 381, 369, 345, 327, 259, 247, 235, 217, 205, 165 *, 123 | 3-Hydroxyphloretin-3',5'-di-C-hexoside |
| − | 613.1772 | 0.5 | C27H33O16 | 493, 475, 433, 403, 373 *, 361, 331, 239, 209 | ||||
| 15 | + | 12.50 | 597.1812 | 283 | −1.2 | C27H33O15 | 289 *, 273, 153 | Eriodictyol-7-O-rutinoside [Eriocitrin] |
| − | 595.1661 | −0.3 | C27H31O15 | 287 *, 151, 135 | ||||
| 16 | + | 13.29 | 595.1647 | 252, 348 | −2.7 | C27H31O15 | 449, 287 * | Luteolin-7-O-rutinoside [Scolymoside] |
| − | 593.1509 | 0.5 | C27H29O15 | 285 * | ||||
| 17 | + | 13.53 | 599.1972 | 284 | −0.7 | C27H35O15 | 479, 461, 449, 431, 419, 407, 395 *, 383, 365, 353, 329, 301, 107 | Phloretin-3',5'-di-C-β-glucoside |
| − | 597.1831 | 2.0 | C27H33O15 | 477, 459, 417, 387, 357 *, 345, 315 | ||||
| 19 | + | 15.85 | 611.197 | 283 | −1.0 | C28H35O15 | 449, 303 *, 177, 153 | Hesperetin-7-O-rutinoside [Hesperidin] |
| − | 609.1837 | 3.0 | C28H33O15 | 301 * |
| Peak a | Mode | tR (min) | Accurate mass b | λmax (nm) | Error (ppm) | Proposed molecular formula | Fragments | Proposed identity |
|---|---|---|---|---|---|---|---|---|
| 2 | + | 3.20 | 345.1186 | 234, 272 | −0.3 | C15H21O9 | 123 *,165 | Unknown |
| − | 343.1024 | −1.5 | C15H19O9 | 163, 119 * | ||||
| 3 | + | 4.20 | 425.1084 | 234, 315 | 0.2 | C19H21O11 | 261, 243, 231, 219, 195 *, 177, 165, 137, 121 | Unknown |
| − | 423.0929 | 0.5 | C19H19O11 | 333, 303, 223, 193 *, 165, 151, 109 | ||||
| 7 | + | 7.83 | nd | 282 | nd | nd | n.d. | Unknown |
| − | 457.1353 | 1.5 | C20H25O12 | 163 *, 119 | ||||
| 8 | + | 7.93 | 597.1812 | 280 | −1.2 | C27H33O15 | 405, 393, 363, 339, 327, 321, 285, 273, 261 *, 219, 207 | Naringenin-di-C-hexoside |
| − | 595.1665 | 0.3 | C27H31O15 | 475, 415, 385 *, 355 | ||||
| 18 | + | 14.68 | 581.1837 | 279 | −5.7 | C27H33O14 | 273 *, 153 | Naringenin-O-dihexoside |
| − | 579.1719 | 0.9 | C27H31O14 | 271 *, 151 |
| Compound | Regression equation | r |
|---|---|---|
| Mangiferin | y = 2089.4x + 7.3 | 1.000 |
| Aspalathin | y = 2351.8x + 1.0 | 1.000 |
| Eriocitrin | y = 1611.6x – 0.3 | 1.000 |
| Hesperidin | y = 1782.1x – 1.8 | 1.000 |
| Luteolin | y = 2781.8x – 4.0 | 1.000 |
| Compound | %RSD (% change) | |||
|---|---|---|---|---|
| Calibration mixture (2 µL) | Calibration mixture (15 µL) | Unfermented extract | Fermented extract | |
| Mangiferin | 0.6 (1.4) | 0.2 (0.4) | 0.9 (−2.7) | 1.9 (−1.7) |
| Aspalathin | 0.8 (−0.9) | 0.4 (−1.1) | - | - |
| Eriocitrin | 0.4 (0.4) | 0.1 (0.2) | 0.8 (−0.2) | 0.7 (−2.3) |
| Hesperidin | 1.1 (−2.1) | 0.6 (−1.8) | 1.2 (−3.3) | 1.0 (−3.5) |
| Luteolin | 0.9 (−1.3) | 0.4 (−1.3) | - | - |
| Isomangiferin | - | - | 0.9 (−1.8) | 1.5 (−4.4) |
| Iriflophenone-3-C-β-glucoside | - | - | 1.2 (−2.3) | 0.6 (−1.4) |
| 3-Hydroxyphloretin-3',5'-di-C-hexoside | - | - | 2.0 (5.5) | 2.9 (9.1) |
| Scolymoside | - | - | 0.9 (−2.3) | 0.7 (−1.7) |
| Phloretin-3',5'-di-C-β-glucoside | - | - | 0.7 (−2.0) | 0.6 (−1.2) |
| Compound | Intra-day (n = 6/day) | Inter-day (n = 3) | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| Calibration mixture (2 µL) | ||||
| Mangiferin | 0.5 | 0.3 | 0.2 | 1.6 |
| Luteolin | 0.3 | 0.6 | 0.5 | 1.2 |
| Hesperidin | 0.8 | 0.6 | 0.8 | 0.8 |
| Eriocitrin | 0.6 | 0.4 | 0.4 | 0.7 |
| Aspalathin | 0.7 | 0.3 | 0.6 | 1.4 |
| Calibration mixture (15 µL) | ||||
| Mangiferin | 0.1 | 0.1 | 0.1 | 1.6 |
| Luteolin | 0.1 | 0.1 | 0.4 | 0.8 |
| Hesperidin | 0.1 | 0.2 | 0.4 | 0.1 |
| Eriocitrin | 0.2 | 0.1 | 0.2 | 0.6 |
| Aspalathin | 0.0 | 0.1 | 0.3 | 1.1 |
| Unfermented C. subternata | ||||
| Iriflophenone-3-C-β-glucoside | 0.2 | 0.4 | 0.1 | 0.3 |
| Mangiferin | 0.1 | 0.1 | 0.1 | 0.1 |
| Isomangiferin | 0.1 | 0.1 | 0.1 | 0.2 |
| 3-Hydroxyphloretin-3',5'-di-C-hexoside | 1.1 | 1.3 | 0.7 | 1.6 |
| Eriocitrin | 1.4 | 1.6 | 1.2 | 2.0 |
| Scolymoside | 0.1 | 0.1 | 0.2 | 0.3 |
| Phloretin-3',5'-di-C-β-glucoside | 0.1 | 0.2 | 0.2 | 0.1 |
| Hesperidin | 0.3 | 0.2 | 0.4 | 0.3 |
| Fermented C. subternata | ||||
| Iriflophenone-3-C-β-glucoside | 0.1 | 0.1 | 0.1 | 0.5 |
| Mangiferin | 0.2 | 0.4 | 0.7 | 1.5 |
| Isomangiferin | 0.2 | 0.1 | 0.1 | 1.2 |
| 3-Hydroxyphloretin-3',5'-di-C-hexoside | 2.0 | 1.7 | 1.8 | 1.6 |
| Eriocitrin | 0.5 | 0.6 | 0.2 | 0.7 |
| Scolymoside | 0.2 | 0.3 | 1.0 | 1.2 |
| Phloretin-3',5'-di-C-β-glucoside | 0.2 | 0.8 | 1.0 | 0.4 |
| Hesperidin | 0.4 | 0.3 | 0.3 | 0.7 |
| Parameter | Leaves | Stems |
|---|---|---|
| Extract yield a | 23.1 ± 2.1 (20.2–27.9) a | 11.8 ± 1.8 (8.6–14.2) b |
| Iriflophenone-3-C-β-glucoside content b | 0.441 ± 0.131 (0.263–0.634) a | 0.286 ± 0.077 (0.180–0.460) b |
| Mangiferin content a | 0.817 ± 0.359 (0.313–1.396) a | 0.373 ± 0.138 (0.188–0.558) b |
| Isomangiferin content a | 0.342 ± 0.104 (0.177–0.489) a | 0.156 ± 0.042 (0.099–0.223) b |
| 3-Hydroxyphloretin-3′,5′-di-C-β-hexoside c | 0.432 ± 0.073 (0.332–0.561) a | 0.493 ± 0.101 (0.311–0.597) a |
| Eriocitrin content a | 0.633 ± 0.177 (0.422–1.003) a | 0.517 ± 0.127 (0.344–0.744) b |
| Scolymoside content d | 0.812 ± 0.454 (0.264–1.443) a | 0.391 ± 0.242 (0.152–0.826) b |
| Phloretin-3′,5′-di-C-β-glucoside e | 0.899 ± 0.252 (0.631–1.364) b | 1.243 ± 0.237 (0.878–1.525) a |
| Hesperidin content a | 0.504 ± 0.495 (0.147–1.517) b | 1.559 ± 0.289 (1.164–1.893) a |
| Total polyphenol content f | 25.5 ± 3.7 (21.1–31.6) a | 21.5 ± 2.2 (18.8–25.8) b |
| TACDPPH g | 2568 ± 234 (2255–2913) a | 2137 ± 233 (1843–2558) b |
| TACORAC g | 9445 ± 822 (7446–10479) a | 8871 ± 518 (8122–9685) a |
| TACFRAP g | 1441 ± 74 (1309–1596) a | 1236 ± 69 (1160–1374) b |
© 2012 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Beer, D.; Schulze, A.E.; Joubert, E.; De Villiers, A.; Malherbe, C.J.; Stander, M.A. Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity. Molecules 2012, 17, 14602-14624. https://doi.org/10.3390/molecules171214602
De Beer D, Schulze AE, Joubert E, De Villiers A, Malherbe CJ, Stander MA. Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity. Molecules. 2012; 17(12):14602-14624. https://doi.org/10.3390/molecules171214602
Chicago/Turabian StyleDe Beer, Dalene, Alexandra E. Schulze, Elizabeth Joubert, André De Villiers, Christiaan J. Malherbe, and Maria A. Stander. 2012. "Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity" Molecules 17, no. 12: 14602-14624. https://doi.org/10.3390/molecules171214602





