New Advances in Titanium-Mediated Free Radical Reactions
Abstract
:1. Introduction
2. Nucleophilic Radical Addition to Imines
2.1. TiCl3/ArN2+ System
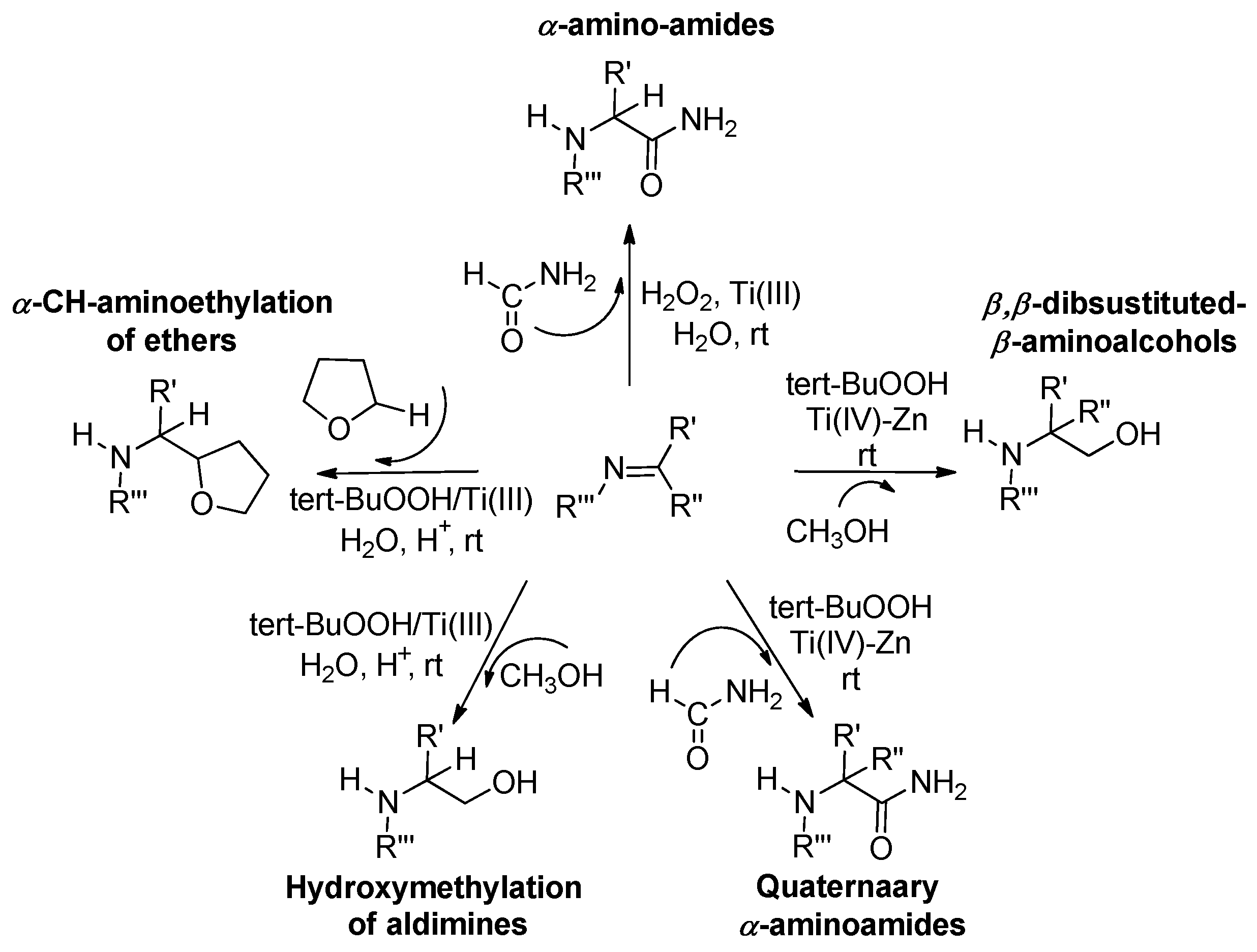
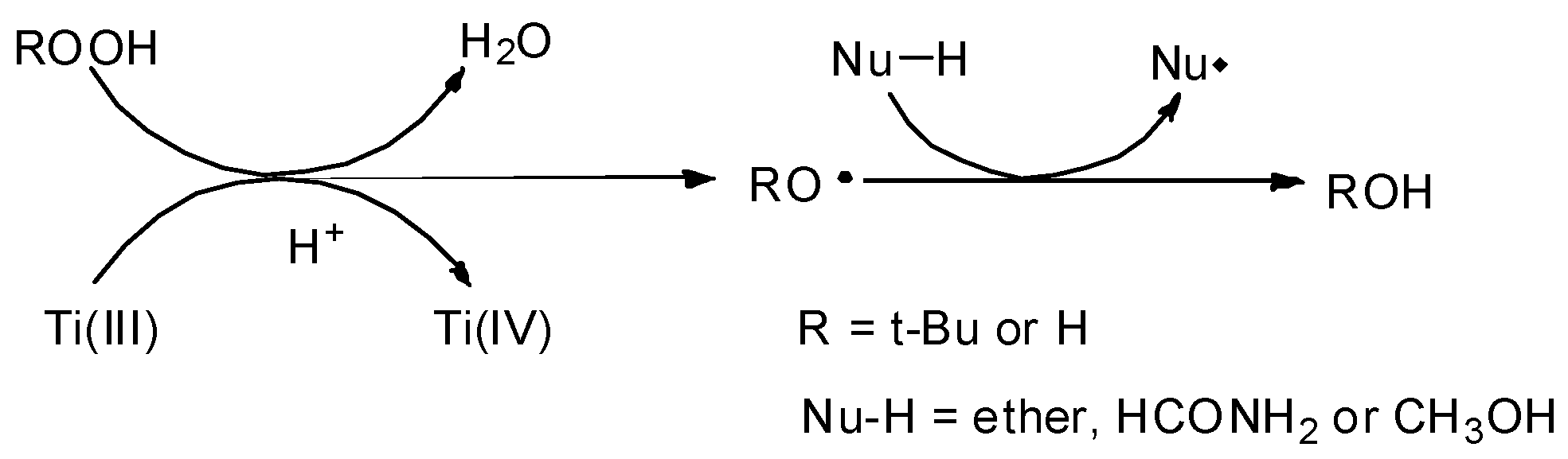
2.2. TiCl3/ROOH and TiCl4-Zn/ROOH Systems
2.2.1. TiCl3/ROOH
2.2.2. TiCl4-Zn/ROOH
2.3. Cp2TiCl2/Zn Systems
3. Coupling Reactions
3.1. Pinacol Reactions
3.2. Allylation Reactions
3.3. Other Coupling Reactions
4. Epoxide Reactions Mediated by Titanocene
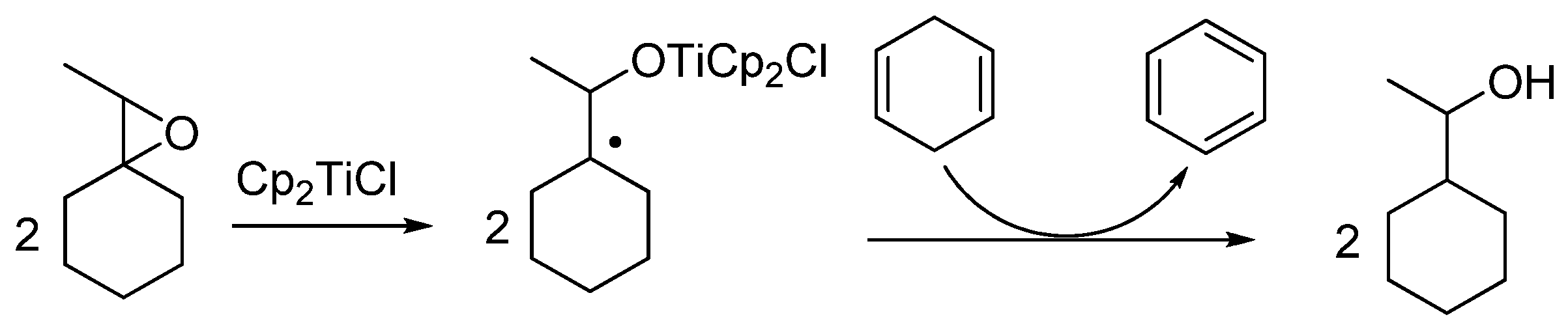
4.1. Epoxide Deoxygenation
4.2. Reductive Epoxide Opening
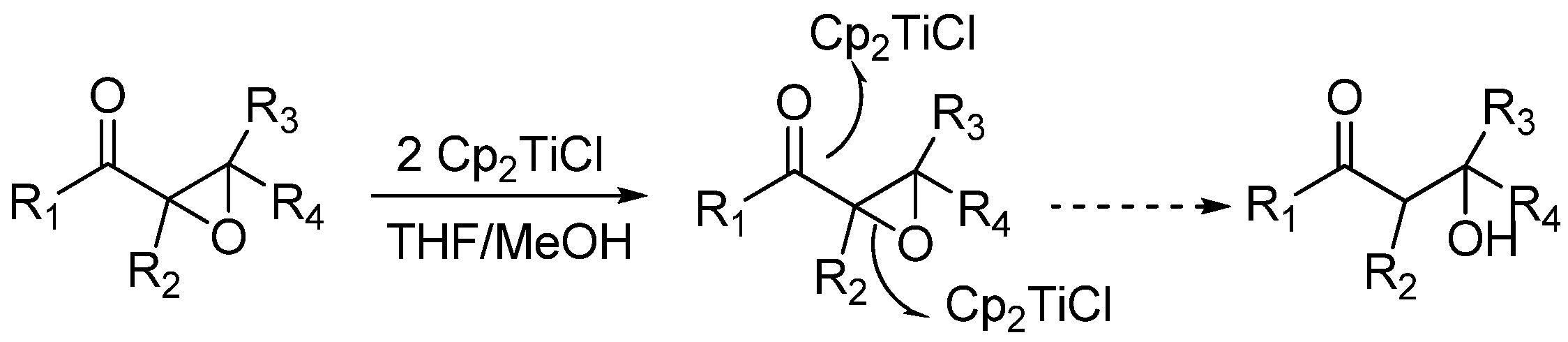
4.2.1. Synthesis of Alcohols
4.2.2. Intermolecular C-C Bond Formation
4.2.3. Intramolecular C-C Bond Formation
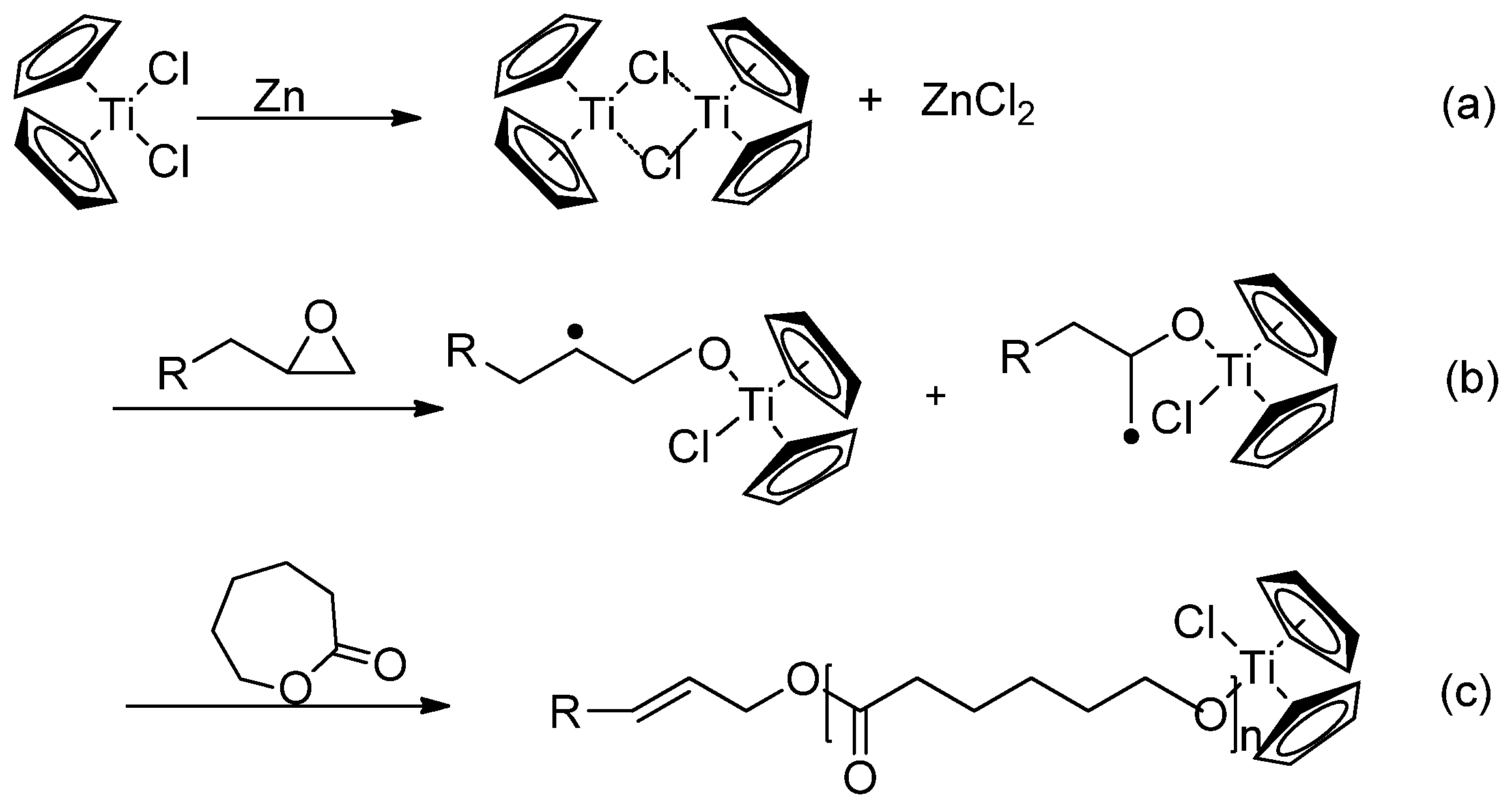
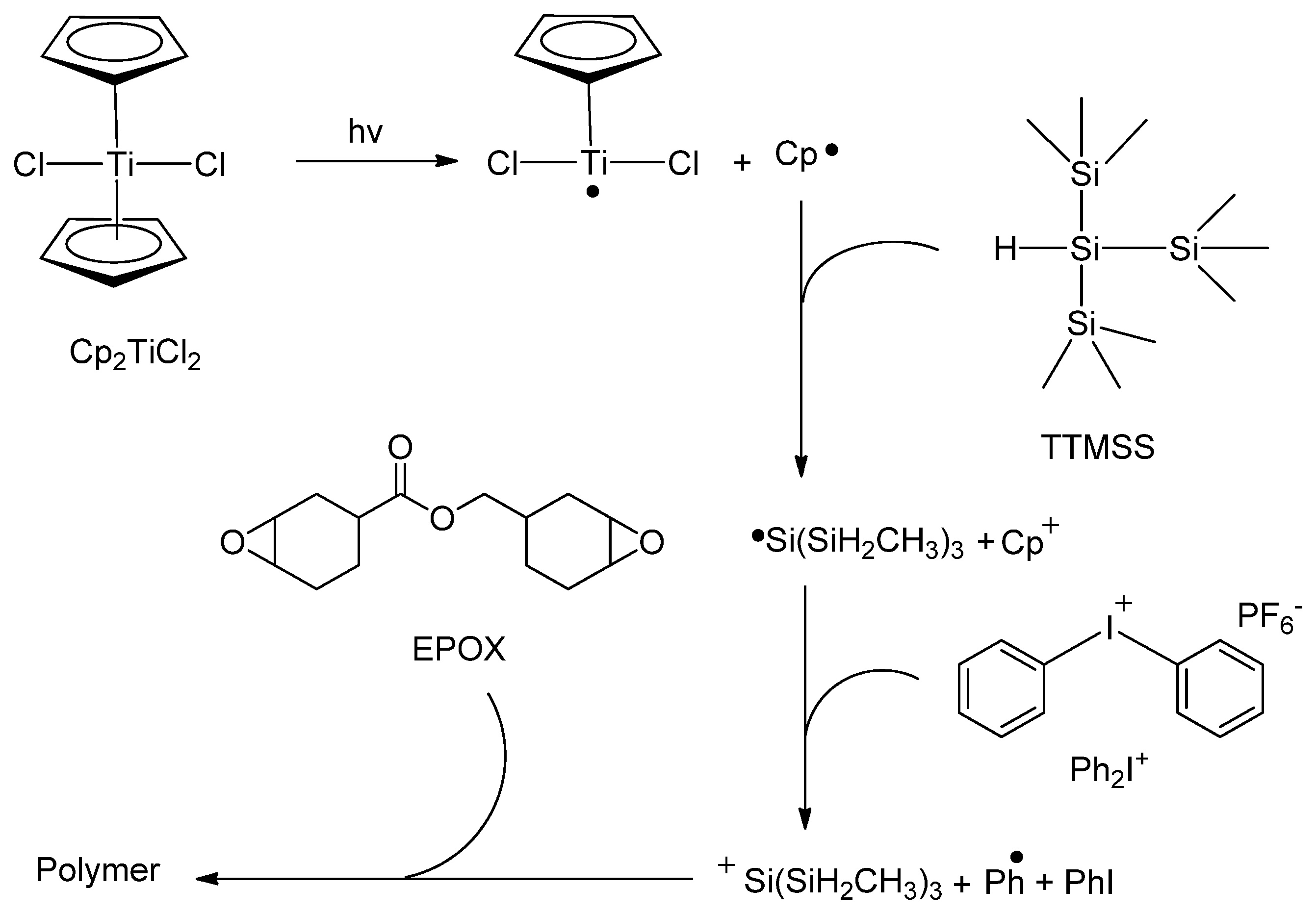
5. Living Polymerizations
6. Conclusions
Acknowledgments
References
- Barton, D.H.; Beaton, J.M.; Geller, L.E.; Pechet, M.M. A new photochemical reaction. J. Am. Chem. Soc. 1960, 82, 2640–2641. [Google Scholar] [CrossRef]
- Renaud, P.; Sibi, M.P. (Eds.) Radicals in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001; Volumes 1 and 2. [Google Scholar]
- Chatgilialoglu, C.; Studer, A. (Eds.) Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Chichester, UK, 2012; Volumes 1 and 2. [Google Scholar]
- Jahn, U. Radicals in transition metal catalyzed reactions? Transition metal catalyzed radical reactions? A fruitful interplay anyway: Part 1. Radical catalysis by group 4 to group 7 elements. Top. Curr. Chem. 2012, 320, 121–189. [Google Scholar] [CrossRef] [PubMed]
- Jahn, U. Radicals in transition metal catalyzed reactions? Transition metal catalyzed radical reactions? A fruitful interplay anyway: Part 2. Radical catalysis by group 8 and 9 elements. Top. Curr. Chem. 2012, 320, 191–322. [Google Scholar] [CrossRef] [PubMed]
- Jahn, U. Radicals in transition metal catalyzed reactions? Transition metal catalyzed radical reactions? A fruitful interplay anyway: Part 3. Radical catalysis by group 10 and 11 elements and bimetallic catalysis. Top. Curr. Chem. 2012, 320, 323–451. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, H. Transition metals and Radicals. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 2, pp. 1003–1018. [Google Scholar]
- Burton, J. Manganese (III) acetate, CAN, and Fe(III) salts in oxidative radical chemistry. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 2, pp. 901–941. [Google Scholar]
- Recupero, F.; Punta, C. Free Radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Chem. Rev. 2007, 107, 3800–3842. [Google Scholar] [CrossRef] [PubMed]
- Minisci, F.; Recupero, F.; Cecchetto, A.; Gambarotti, C.; Punta, C.; Faletti, R.; Paganelli, R.; Pedulli, G.F. Mechanisms of the aerobic oxidation of alcohols to aldehydes and ketones, catalysed under mild conditions by persistent and non-persistent nitroxyl radicals and transition metal salts - Polar, enthalpic, and captodative effects. Eur. J. Org. Chem. 2004, 109–119. [Google Scholar] [CrossRef]
- Coote, S.C.; Flowers, R.A., II; Skrydstrup, T.; Procter, D.J. Organic synthesis using samarium diiodide. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 2, pp. 849–900. [Google Scholar]
- Friestad, G.K. Asymmetric radical addition to chiral hydrazones. In Topics in Current Chemistry: Radicals in Synthesis III; Gansauer, A., Heinrich, M., Eds.; Springer-Verlag: Berlin, Germany, 2012; Volume 320, pp. 61–92. [Google Scholar]
- Friestad, G.K. Organomanganese-mediated radical reactions. In The Chemistry of Organo-Manganese Compounds; Marek, I., Rappoport, Z., Eds.; Wiley: Chichester, UK, 2011; pp. 559–584. [Google Scholar]
- Yamada, K.; Tomioka, K. Copper-catalyzed asymmetric alkylation of imines with dialkylzinc and related reactions. Chem. Rev. 2008, 108, 2874–2886. [Google Scholar] [CrossRef] [PubMed]
- Akindele, T.; Yamada, K.; Tomioka, K. Dimethylzinc-initiated radical reactions. Acc. Chem. Res. 2009, 42, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Pastori, N.; Gambarotti, C.; Punta, C. Recent developments in nucleophilic radical addition to imines: The key role of transition metals and the new Porta radical-type version of the Mannich and Strecker Reactions. Mini-Rev. Org. Chem. 2009, 6, 184–195. [Google Scholar] [CrossRef]
- Miyabe, H.; Yoshioka, E.; Kohtani, S. Progress in intermolecular carbon radical addition to imine derivatives. Curr. Org. Chem. 2010, 14, 1254–1264. [Google Scholar] [CrossRef]
- Rowlands, G.J. Radicals in organic synthesis. Part 1. Tetrahedron 2009, 65, 8603–8655. [Google Scholar] [CrossRef]
- Rowlands, G.J. Radicals in organic synthesis. Part 2. Tetrahedron 2010, 66, 1593–1636. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Terashima, T.; Sawamoto, M. Transition metal-catalyzed living radical polymerization: toward perfection in catalysis and precision polymer synthesis. Chem. Rev. 2009, 109, 4963–5050. [Google Scholar] [CrossRef] [PubMed]
- Pintauer, T.; Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 2008, 37, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Gambarotti, C.; Punta, C.; Recupero, F.; Caronna, T.; Palmisano, L. TiO2 in organic photosynthesis: Sunlight induced functionalization of heterocyclic bases. Curr. Org. Chem. 2010, 14, 1153–1169. [Google Scholar] [CrossRef]
- Fagnoni, M.; Dondi, D.; Ravelli, D.; Albini, A. Photocatalysis for the formation of the C-C bond. Chem. Rev. 2007, 107, 2725–2756. [Google Scholar] [CrossRef] [PubMed]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Org. 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Friestad, G.K.; Ji, A.; Baltrusaitis, J.; Korapala, C.S.; Qin, J. Scope of stereoselective Mn-mediated radical addition to chiral hydrazones and application in a formal synthesis of quinine. J. Org. Chem. 2012, 77, 3159–3180. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, H.; Nagai, K.; Tomioka, K. Copper-amidophosphine catalyst in asymmetric addition of organozinc to Imines. J. Am. Chem. Soc. 2000, 122, 12055–12056. [Google Scholar] [CrossRef]
- Nagai, K.; Fujihara, H.; Kuriyama, M.; Yamada, K.; Tomioka, K. Efficient chiral amidophosphine ligand for copper-catalyzed asymmetric addition of diethylzinc to N-sulfonylimines. Chem. Lett. 2002, 31, 8–9. [Google Scholar] [CrossRef]
- Soeta, T.; Nagai, K.; Fujihara, H.; Kuriyama, M.; Tomioka, K. Asymmetric alkylation of N-toluenesulfonylimines with dialkylzinc reagents catalyzed by copper-chiral amidophosphine. J. Org. Chem. 2003, 68, 9723–9727. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Traverse, J.F.; Hoveyda, A.H.; Snapper, M.L. Enantioselective synthesis of arylamines through Zr-catalyzed addition of dialkylzincs to imines. Reaction development by screening of parallel libraries. J. Am. Chem. Soc. 2001, 123, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Traverse, J.F.; Hoveyda, A.H.; Snapper, M.L. Three-component catalytic asymmetric synthesis of aliphatic amines. J. Am. Chem. Soc. 2001, 123, 10409–10410. [Google Scholar] [CrossRef] [PubMed]
- Akullian, L.C.; Snapper, M.L.; Hoveyda, A.H. Three-component enantioselective synthesis of propargylamines through Zr-catalyzed additions of alkyl zinc reagents to alkynylimines. Angew. Chem. Int. Ed. 2003, 42, 4244–4247. [Google Scholar] [CrossRef] [PubMed]
- Clerici, A.; Porta, O. Arylative amination of aldehydes promoted by aqueous titanium trichloride. Tetrahedron Lett. 1990, 31, 2069–2072. [Google Scholar] [CrossRef]
- Cannella, R.; Clerici, A.; Pastori, N.; Regolini, E.; Porta, O. One-pot four-component reaction: Aqueous TiCl3/PhN2+-mediated alkyl radical addition to imines generated in situ. Org. Lett. 2005, 7, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Clerici, A.; Cannella, R.; Panzeri, W.; Pastori, N.; Regolini, E.; Porta, O. TiCl3/PhN2+-mediated radical addition of ethers to aldimines generated in situ under aqueous conditions. Tetrahedron Lett. 2005, 46, 8351–8354. [Google Scholar] [CrossRef]
- Clerici, A.; Cannella, R.; Panzeri, W.; Porta, O. A Free radical Mannich type reaction: selective α-CH-aminomethylation of ethers by Ti(III)/t-BuOOH system under aqueous acidic conditions. Tetrahedron 2006, 62, 5986–5994. [Google Scholar] [CrossRef]
- Cannella, R.; Clerici, A.; Panzeri, W.; Pastori, N.; Punta, C.; Porta, O. Free-radical version of Strecker Synthesis of α-aminomides promoted by aqueous H2O2/TiCl3/HCONH2 System. J. Am. Chem. Soc. 2006, 128, 5358–5359. [Google Scholar] [CrossRef] [PubMed]
- Clerici, A.; Ghilardi, A.; Pastori, N.; Punta, C. A new one-pot, four-component synthesis of 1,2-amino alcohols: TiCl3/t-BuOOH-mediated radical hydroxymethylation of imines. Org. Lett. 2008, 10, 5063–5066. [Google Scholar] [CrossRef] [PubMed]
- Spaccini, R.; Ghilardi, A.; Pastori, N.; Clerici, A.; Porta, O.; Punta, C. Efficient radical domino approach to β-aminoalcohols from arylamines and alcohols triggered by Ti(III)/t-BuOOH. Tetrahedron 2010, 66, 2044–2052. [Google Scholar] [CrossRef]
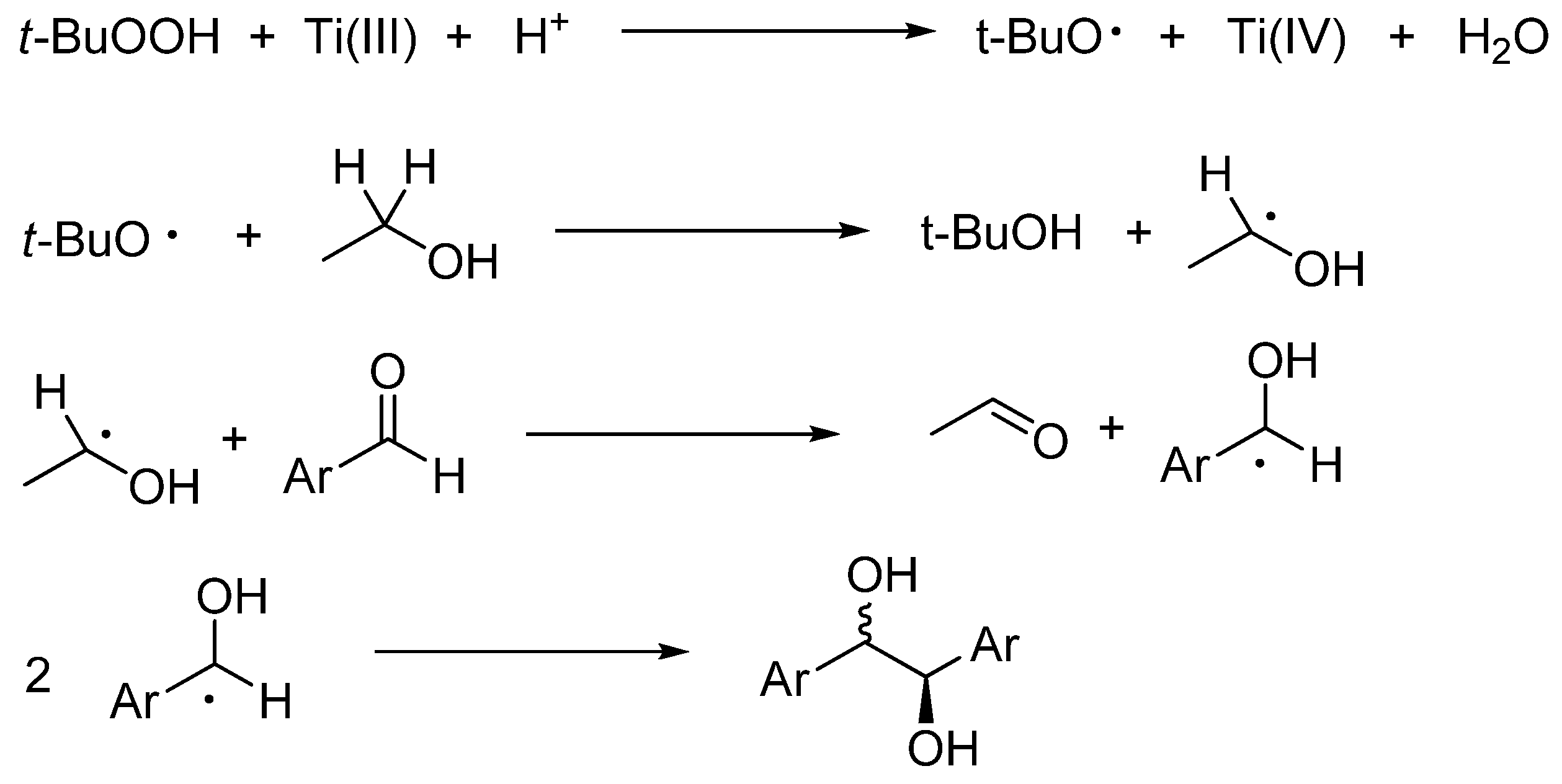
- Clerici, A.; Greco, C.; Panzeri, W.; Pastori, N.; Punta, C.; Porta, O. Reductive coupling of aromatic aldehydes promoted by an aqueous TiCl3/t-BuOOH system in alcoholic cosolvents. Eur. J. Org. Chem. 2007, 4050–4055. [Google Scholar] [CrossRef]
- Pastori, N.; Greco, C.; Clerici, A.; Punta, C.; Porta, O. Free-Radical addition to ketimines generated in situ. One-pot synthesis of quaternary α-aminomides promoted by a H2O2/TiCl4-Zn/HCONH2 system. Org. Lett. 2010, 12, 3898–3901. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Pastori, N.; Clerici, A.; Punta, C. Free-radical hydroxymethylation of ketimines generated in situ: A one-pot multicomponent synthesis of β,β-disubstituted-β-aminoalcohols. Tetrahedron 2012, 68, 10151–10156. [Google Scholar] [CrossRef]
- Prosperini, S.; Pastori, N.; Ghilardi, A.; Clerici, A.; Punta, C. New domino radical synthesis of aminoalcohols promoted by TiCl4–Zn/t-BuOOH system: selective hydroxyalkylation of amines in alcohol or in cyclic ether cosolvents. Org. Biomol. Chem. 2011, 9, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Roy, S.C. Titanocene(III) chloride mediated radical induced allylation of aldimines: Formal synthesis of C-linked 40-deoxy aza-disaccharide. J. Org. Chem. 2011, 76, 7229–7234. [Google Scholar] [CrossRef] [PubMed]
- Ramόn, D.J.; Yus, M. In the arena of enantioselective synthesis, titanium complexes wear the laurel wreath. Chem. Rev. 2006, 106, 2126–2208. [Google Scholar] [CrossRef] [PubMed]
- Szymoniak, J.; Mose, C. Synthesis and reactivity of allyltitanium derivatives. In Titanium and Zirconium in Organic Synthesis; Marek, I., Ed.; Wiley-VCH: Weinheim, Germany, 2002; pp. 451–474. [Google Scholar]
- Chatterjee, A.; Joshi, N.N. Evolution of the stereoselective pinacol coupling reaction. Tetrahedron 2006, 62, 12137–12158. [Google Scholar] [CrossRef]
- Clerici, A.; Clerici, L.; Porta, O. A highly dl-stereoselective pinacolization of aromatic aldehydes mediated by titanium trichloride in dichloromethane. Tetrahedron Lett. 1996, 37, 3035–3038. [Google Scholar] [CrossRef]
- Clerici, A.; Pastori, N.; Porta, O. On the paradox of TiCl4 reducing power: pinacol coupling and two-carbon homologation of carbonyl compounds. Tetrahedron Lett. 2004, 45, 1825–1827. [Google Scholar] [CrossRef]
- Paradas, M.; Campaña, A.G.; Estévez, R.E.; de Cienfuegos, L.A.; Jiménez, T.; Robles, R.; Cuerva, J.M.; Oltra, J.E. Unexpected TiIII/Mn-promoted pinacol coupling of ketones. J. Org. Chem. 2009, 74, 3616–3619. [Google Scholar] [CrossRef] [PubMed]
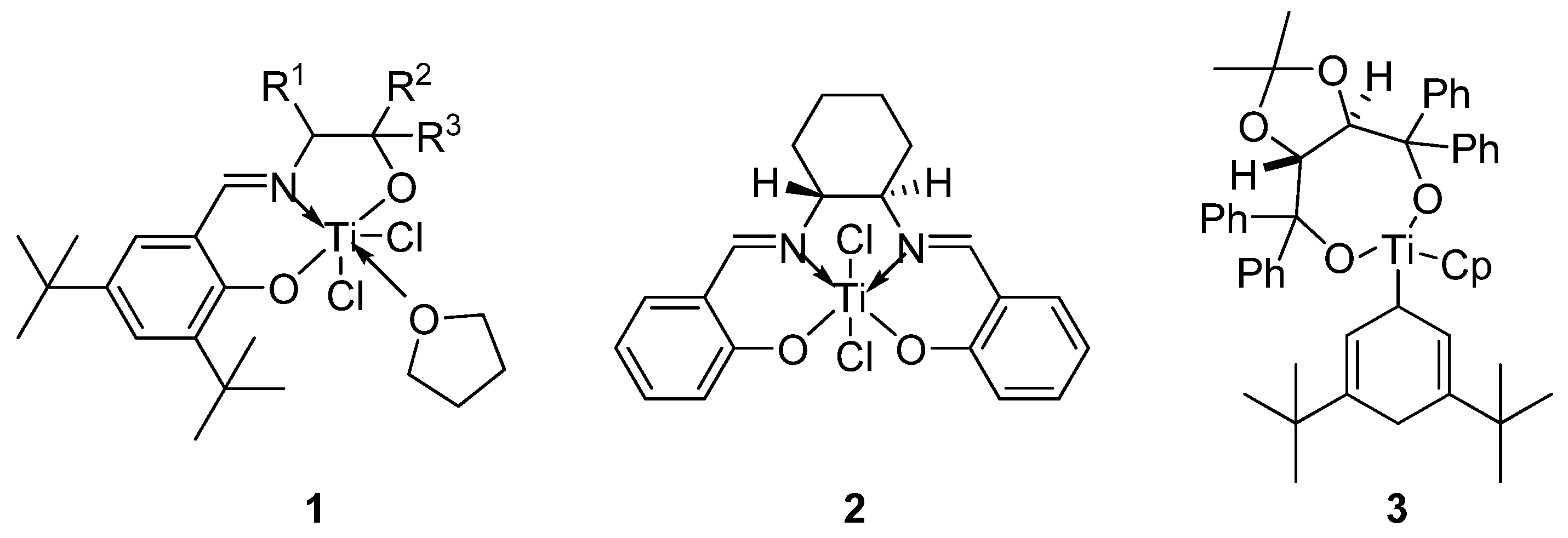
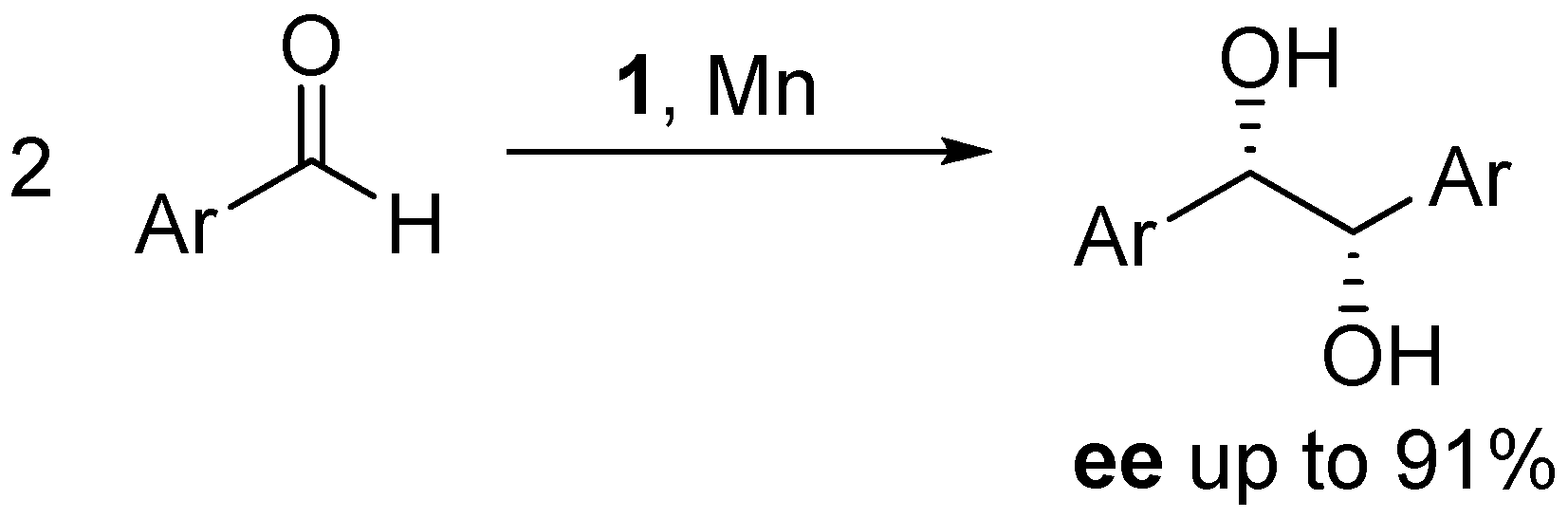
- Bensari, A.; Renaud, J.-L.; Riant, O. Enantioselective pinacol coupling of aldehydes mediated and catalyzed by chiral titanium complexes. Org. Lett. 2001, 3, 3863–3865. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Bennur, T.H.; Joshi, N.N. Truly catalytic and enantioselective pinacol coupling of aryl aldehydes mediated by chiral Ti(III) complexes. J. Org. Chem. 2003, 68, 5668–5671. [Google Scholar] [CrossRef] [PubMed]
- Knoop, C.A.; Studer, A. A new method for the generation of Ti(III) complexes and its application in pinacol coupling reactions. Adv. Synth. Catal. 2005, 347, 1542–1546. [Google Scholar] [CrossRef]
- Sancho-Sanz, I.; Miguel, D.; Millán, A.; Estévez, R.E.; Oller-López, J.L.; Álvarez-Manzaneda, E.; Robles, R.; Cuerva, J.M.; Justicia, J. Titanocene(III)-promoted Barbier-type crotylation of carbonyl compounds. J. Org. Chem. 2011, 76, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Estévez, R.E.; Justicia, J.; Bazdi, B.; Fuentes, N.; Paradas, M.; Choquesilo-Lazarte, D.; Garcia-Ruiz, J.M.; Robles, R.; Gansäuer, A.; Cuerva, J.M.; Oltra, J.E. Ti-catalyzed Barbier-type allylations and related reactions. Chem. Eur. J. 2009, 15, 2774–2791. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, T.; Morcillo, S.P.; Martin-Lasanta, A.; Collado-Sanz, D.; Cárdenas, D.J.; Justicia, J.; Cuerva, J.M. Combining the power of TiIII-mediated processes for easy access to hydroxylated polycyclic terpenoids: Synthesis of sesterstatin 1 and C–D rings of aspergilloxide. Chem. Eur. J. 2012, 18, 12825–12833. [Google Scholar]
- Campaña, A.G.; Bazdi, B.; Fuentes, N.; Robles, R.; Cureva, J.M.; Oltra, J.E.; Porcel, S.; Echavarren, A.M. Divergent Titanium-Mediated Allylations with Modulation by Nickel or Palladium. Angew. Chem. Int. Ed. 2008, 47, 7625–7629. [Google Scholar] [CrossRef]
- Millán, A.; Campaña, A.G.; Bazdi, B.; Miguel, D.; de Cienfuegos, L.A.; Echavarren, A.M.; Cuerva, J.M. Ti/Pd bimetallic systems for the efficient allylation of carbonyl compounds and homocoupling reactions. Chem. Eur. J. 2011, 17, 3985–3994. [Google Scholar] [CrossRef] [PubMed]
- Millán, A.; de Cienfuegos, L.A.; Martin-Lasanta, A.; Campaña, A.G.; Cuerva, J.M. Titanium/Palladium-mediated regioselective propargylation of ketones using propargylic carbonates as pronucleophiles. Adv. Synth. Catal. 2011, 353, 73–78. [Google Scholar] [CrossRef]
- Millán, A.; Martin-Lasanta, A.; Miguel, D.; de Cienfuegos, L.A.; Cuerva, J.M. Ti/Pd-promoted intramolecular Michael-type addition of allylic carboxylates to activated alkenes. Chem. Commun. 2011, 47, 10470–10472. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peragón, Á.; Millán, A.; Campaña, A.G.; Rodríguez-Márquez, I.; Resa, S.; Miguel, D.; de Cienfuegos, L.A.; Cuerva, J.M. Ti/Ni-based multimetallic system for the efficient allylation of carbonyl compounds. Eur. J. Org. Chem. 2012, 1499–1503. [Google Scholar] [CrossRef]
- Mandal, S.K.; Paira, M.; Roy, S.C. Titanium(III) chloride mediated synthesis of furan derivatives: Synthesis of (±)-evodone. J. Chem. Sci. 2010, 122, 423–426. [Google Scholar] [CrossRef]
- Saha, S.; Mandal, S.K.; Roy, S.C. Titanocene(III) chloride mediated radical induced asymmetric synthesis of α-methylene bis-γ-butyrolactone. Tetrahedron Lett. 2011, 52, 3128–3130. [Google Scholar] [CrossRef]
- Rosales, A.; Muñoz-Bascón, J.; López-Sánchez, C.; Álvarez-Corral, M.; Muñoz-Dorado, M.; Rodríguez-García, I.; Oltra, J.E. Ti-catalyzed homolytic opening of ozonides: A sustainable C−C bond-forming reaction. J. Org. Chem. 2012, 77, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Streuff, J.; Feurer, M.; Bichovski, P.; Frey, G.; Gellrich, U. Enantioselective Titanium(III)-catalyzed reductive cyclization of ketonitriles. Angew. Chem. Int. Ed. 2012, 51, 8661–8664. [Google Scholar] [CrossRef] [PubMed]
- Griller, D.; Ingold, K.U. Persistent carbon-centered radicals. Acc. Chem. Res. 1976, 9, 13–19. [Google Scholar] [CrossRef]
- Spaccini, R.; Pastori, N.; Clerici, A.; Punta, C.; Porta, O. Key role of Ti(IV) in the selective radical-radical cross-coupling mediated by the Ingold-Fischer effect. J. Am. Chem. Soc. 2008, 130, 18018–18024. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Justicia, J.; Fan, C.-A.; Worgull, D.; Piestert, F. Reductive C-C bond formation after opening via electron transfer. Top. Curr. Chem. 2007, 279, 25–52. [Google Scholar]
- Gansäuer, A.; Lauterbach, T.; Narayan, S. Strained heterocycles in radical chemistry. Angew. Chem. Int. Ed. 2003, 42, 5556–5573. [Google Scholar] [CrossRef] [PubMed]
- Daasbjerg, K.; Svith, H.; Grimme, S.; Gerenkamp, M.; Muck-Lichtenfeld, C.; Gansäuer, A.; Barchuk, A.; Keller, F. Elucidation of the mechanism of titanocene-mediated epoxide opening by a combined experimental and theoretical approach. Angew. Chem. Int. Ed. 2006, 45, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Barchuk, A.; Keller, F.; Schmitt, M.; Grimme, S.; Gerenkamp, M.; Muck-Lichtenfeld, C.; Daasbjerg, K.; Svith, H. Mechanism of titanocene-mediated epoxide opening through homolytic substitution. J. Am. Chem. Soc. 2007, 129, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- RajanBabu, T.V.; Nugent, W.A. Intermolecular addition of epoxides to activated olefins: a new reaction. J. Am. Chem. Soc. 1989, 111, 4525–4527. [Google Scholar] [CrossRef]
- RajanBabu, T.V.; Nugent, W.A.; Beattie, M.S. Free radical-mediated reduction and deoxygenation of epoxides. J. Am. Chem. Soc. 1990, 112, 6408–6409. [Google Scholar] [CrossRef]
- Cuerva, J.M.; Juan, J.C.; Justicia, J.; Oller-Lòpez, J.L.; Oltra, J.E. Cp2TiCl in natural product synthesis. Top. Curr. Chem. 2006, 264, 63–91. [Google Scholar]
- Gansäuer, A.; Fleckhaus, A. Epoxides in titanocenes-mediated and -catalyzed radical reactions. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 2, pp. 989–1001. [Google Scholar]
- Fernández-Mateos, A.; Madrazo, S.E.; Teijón, P.H.; González, R.R. Titanocene-promoted eliminations on epoxy alcohols and epoxy esters. Eur. J. Org. Chem. 2010, 856–861. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; Teijón, P.H. Radical reactions on pinene-oxide derivatives induced by Ti(III). Tetrahedron 2011, 67, 9529–9534. [Google Scholar]
- Hardouin, C.; Doris, E.; Rousseau, B.; Mioskowski, C. Concise synthesis of anhydrovinblastine from leurosine. Org. Lett. 2002, 4, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Quìlez del Moral, J.F.; Sànchez, E.M.; Arteaga, J.F. Regio- and diastereo-selective reductive coupling of vinylepoxides catalyzed by titanocene chloride. Org Lett. 2006, 8, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Aldegunge, M.J.; Astedo, L.; Granja, J.R. Synthesis of 2-ene-1,4-diols by a new cascade-opening of 1,3-diepoxides: towards an efficient synthesis of dihydroxytaxoid derivatives. Chem. Eur. J. 2009, 15, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; Jimenez, T.; Morcillo, S.P.; Cuerva, J.M.; Oltra, J.E. Mixed disproportionation versus radical trapping in titanocene (III)-promoted epoxide openings. Tetrahedron 2009, 65, 10837–10841. [Google Scholar] [CrossRef]
- Barrero, A.F.; Rosales, A.; Cuerva, J.M.; Oltra, J.E. Unified synthesis of eudesmanolides, combining biomimetic strategies with homogeneous catalysis and free-radical chemistry. Org. Lett. 2003, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; Rosales, B.E.; Oller-Lopez, J.L.; Valdivia, N.; Haidour, A.; Oltra, J.E.; Barrero, A.F.; Cardenas, D.J.; Cuerva, J.M. Titanocene-catalyzed cascade cyclization of epoxypolyprenes: Straightforward synthesis of terpenoids by free-radical chemistry. Chem. Eur. 2004, 10, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; Oltra, J.E.; Cuerva, J.M. Exploiting Pd-II and Ti-III chemistry to obtain gamma-dioxygenated terpenoids: Synthesis of rostratone and novel approaches to aphidicolin and pyripyropene A. J. Org. Chem. 2005, 70, 8265–8272. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Worgull, D.; Justicia, J. Catalytic epoxypolyene cyclization via radicals: A simple total synthesis of sclareol oxide and its 8-epimer. Synthesis-Stuttgart 2006, 2151–2154. [Google Scholar]
- Justicia, J.; Campanã, G.A.; Bazdi, B.; Robles, R.; Cuerva, J.M.; Oltra, J.E. Titanium-catalyzed enantioselective synthesis of alpha-ambrinol. Adv. Synth. Catal. 2008, 350, 571–576. [Google Scholar] [CrossRef]
- Gansäuer, A.; Bluhm, H. Reagent-controlled transition-metal-catalyzed radical reactions. Chem. Rev. 2000, 100, 2771–2788. [Google Scholar] [CrossRef] [PubMed]
- Cuerva, J.M.; Campanã, A.G.; Justicia, J.; Rosales, A.; Oller-Lopez, J.L.; Robles, R.; Cardenas, D.J.; Bunuel, E.; Oltra, J.E. Water: The ideal hydrogen-atom source in free-radical chemistry mediated by Ti-III and other single-electron-transfer metals? Angew. Chem. Int. Ed. 2006, 45, 5522–5526. [Google Scholar] [CrossRef] [PubMed]
- Paradas, M.; Campaña, A.G.; Marcos, M.L.; Justicia, J.; Haidour, A.; Robles, R.; Cárdenas, D.J.; Oltra, E.J.; Cuerva, J.M. Unprecedented H-atom transfer from water to ketyl radicals mediated by Cp2TiCl. Dalton Trans. 2010, 39, 8796–8800. [Google Scholar] [CrossRef] [PubMed]
- Paradas, M.; Campaña, A.G.; Jiménez, T.; Robles, R.; Oltra, J.E.; Buñuel, E.; Justicia, J.; Cárdenas, D.J.; Cuerva, J.M. Understanding the exceptional hydrogen-atom donor characteristics of water in TiIII- mediated free-radical chemistry. J. Am. Chem. Soc. 2010, 132, 12748–12756. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, T.; Campaña, A.G.; Bazdi, B.; Paradas, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Oltra, J.E.; Robles, R.; Justicia, J.; Cuerva, J.M. Radical reduction of epoxides using a titanocene (III) water system: synthesis of β-deuterated alcohols and their use as internal standards in food analysis. Eur. J. Org. Chem. 2010, 4288–4295. [Google Scholar] [CrossRef]
- Gansäuer, A.; Otte, M.; Shi, L. Radical cyclizations terminated by Ir-catalyzed hydrogen atom transfer. J. Am. Chem. Soc. 2011, 133, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Fan, C.A.; Piester, F. Sustainable radical reduction through catalytic hydrogen atom transfer. J. Am. Chem. Soc. 2008, 130, 6916–6917. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Narayan, S. Titanocene-catalysed electron transfer-mediated opening of epoxides. Adv. Synth. Cat. 2002, 344, 465–475. [Google Scholar] [CrossRef]
- Gansäuer, A.; Bluhm, H.; Lauterbach, T. Titanocene-catalysed enantioselective opening of meso-epoxides. Adv. Synth. Catal. 2001, 343, 785–787. [Google Scholar] [CrossRef]
- Gansäuer, A.; Bluhm, H.; Rinker, B.; Narayan, S.; Schick, M.; Lauterbach, T.; Pierobon, M. Reagent-controlled stereoselectivity in titanocene-catalyzed epoxide openings: Reductions and intermolecular additions to alpha,beta-unsaturated carbonyl compounds. Chem. Eur. J. 2003, 9, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Narayan, S.; Schiffer-Ndene, N.; Bluhm, H.; Oltra, J.E.; Cuerva, J.M.; Rosales, A.; Nieger, M. An improved synthesis of Kagan’s menthyl substituted titanocene and zirconocene dichloride, comparison of their crystal structures, and preliminary catalyst evaluation. J. Organomet. Chem. 2006, 691, 2327–2331. [Google Scholar] [CrossRef]
- Gansäuer, A.; Bluhm, H.; Pierobon, M.; Keller, M. Conformational preferences of titanocene dichlorides with ligands derived from menthol: Comparison of structures in solution and in the crystal. Organometallics 2001, 20, 914–919. [Google Scholar] [CrossRef]
- Gansäuer, A.; Fan, C.-A.; Keller, F.; Karbaum, P. Regiodivergent epoxide openining: A concept in stereoselective catalysis beyond classical kinetic resolution and desymmetrizations. Chem. Eur. J. 2007, 13, 8084–8090. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Shi, L.; Otte, M. Catalytic enantioselective radical cyclization via regiodivergent epoxide opening. J. Am. Chem. Soc. 2010, 132, 11858–11859. [Google Scholar] [CrossRef] [PubMed]
- Gansauer, A.; Fan, C.-A.; Keller, F.; Keil, J. Titanocene-catalyzed regiodivergent epoxide openings. J. Am. Chem. Soc. 2007, 129, 3484–3485. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Rosales, A.; Cuerva, J.M.; Gansäuer, A.; Oltra, J.E. Titanocene-catalyzed, selective reduction of ketones in aqueous media. A safe, mild, inexpensive procedure for the synthesis of secondary alcohols via radical chemistry. Tetrahedron Lett. 2003, 44, 1079–1082. [Google Scholar] [CrossRef]
- Oller-López, J.L.; Campaña, A.G.; Cuerva, J.M.; Oltra, J.E. Aromatic carbonyl compound reduction and pinacol coupling processes mediated by titanocene(III)/Zn in water. Synthesis 2005, 2619–2622. [Google Scholar]
- Chakraborty, T.K.; Das, S. Synthesis of chiral 1,3-diols by radical-mediated regioselective opening of 2,3-epoxy alcohols using Cp(2)TiCl. Tetrahedron Lett. 2002, 43, 2313–2315. [Google Scholar] [CrossRef]
- Hardouin, C.; Chevallier, F.; Rousseau, B.; Doris, E. Cp2TiCl-mediated selective reduction of alpha,beta-epoxy ketones. J. Org. Chem. 2001, 66, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, F.; Sandoval, C. Cp2TiCl-promoted isomerization of trisubstituted epoxides to exo-methylene allylic alcohols on carvone derivatives. J. Org. Chem. 2004, 69, 5275–5280. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Cuerva, J.M.; Alvarez-Manzaneda, E.J.; Oltra, J.E.; Chahboun, R. First synthesis of achilleol A using titanium(III) chemistry. Tetrahedron Lett. 2002, 43, 2793–2796. [Google Scholar] [CrossRef]
- Barrero, A.F.; Cuerva, J.M.; Herrador, M.M.; Valdivia, M.V. A new strategy for the synthesis of cyclic terpenoids based on the radical opening of acyclic epoxypolyenes. J. Org. Chem. 2001, 66, 4074–4078. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Cuerva, J.M.; Rosales, A. Effects of solvents and water in Ti(III)-mediated radical cyclizations of epoxygermacrolides. Straightforward synthesis and absolute stereochemistry of (+)-3α-hydroxyreynosin and related eudesmanolides. J. Org. Chem. 2002, 67, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Kamoshita, M.; Doi, T.; Yamada, H.; Takahashi, T. Stereo- and regio-selective Ti-mediated radical cyclization of epoxy-alkenes: Synthesis of the A and C ring synthons of paclitaxel. Tetrahedron Lett. 2001, 42, 7855–7857. [Google Scholar] [CrossRef]
- Gansäuer, A.; Klatte, M.; Brändle, G.M.; Friedrich, J. Catalytic hydrogen atom transfer (HAT) for sustainable and diastereoselective radical reduction. Angew. Chem. Int. Ed. 2012, 51, 8891–8894. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.K.; Samanta, R.; Das, S. Radical-mediated opening of 2,3-epoxy alcohols using Cp2TiCl: Stereoselective construction of quaternary chiral centers. J. Org. Chem. 2006, 71, 3321–3324. [Google Scholar] [CrossRef] [PubMed]
- Dötz, K.H.; Gomes da Silva, E. Carbohydrate-modified fused pyranosylidene complexes via radical addition of epoxides to unsaturated metal carbenes. Tetrahedron 2000, 56, 8291–8299. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; Martín de la Nava, E.; Pascual Coca, G.; Ramos Silvo, A.; Rubio González, R. Radicals from epoxides. Intramolecular addition to aldehyde and ketone carbonyls. Org. Lett. 1999, 1, 607–609. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; Mateos Burón, I.; Rabanedo Clemente, R.; Ramos Silvo, A.I.; Rubio González, R. Radical cyclization of epoxynitriles mediated by titanocene chloride. Synlett 2004, 1011–1014. [Google Scholar] [CrossRef]
- Gansäuer, A.; Lauterbach, T.; Geich-Gimbel, D. Polarity matching of radical trapping: High yielding 3-exo and 4-exo cyclizations. Chem. Eur. J. 2004, 10, 4983–4990. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, A.; Mateos Burón, L.; Martín de la Nava, E.M.; Rabanedo Clemente, R.; Rubio González, R.; Sanz González, F. Effect of tether length on Ti(III)-mediated cyclization of epoxyalkenes and unsaturated epoxyketones. Synlett 2004, 2553–2557. [Google Scholar] [CrossRef]
- Friedrich, J.; Dolg, M.; Gansäuer, A.; Geich-Gimbel, D.; Lauterbach, T. A combined theoretical and experimental study of efficient and fast titanocene-catalyzed 3-exo cyclizations. J. Am. Chem. Soc. 2005, 127, 7071–7077. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; de Cienfuegos, L.A.; Campaña, A.G.; Miguel, D.; Jakoby, V.; Gansäuer, A.; Cuerva, J.M. Bioinspired terpene synthesis: a radical approach. Chem. Soc. Rev. 2011, 40, 3525–3537. [Google Scholar] [CrossRef] [PubMed]
- Ruano, G.; Grande, M.; Anaya, J.J. Stereospecific synthesis of highly functionalized tricyclic beta-lactams by radical cyclizations using titanocene monochloride. J. Org. Chem. 2002, 67, 8243–8246. [Google Scholar] [CrossRef] [PubMed]
- Ruano, G.; Matiánez, J.; Grande, M. Stereospecific synthesis of polyfunctionalized carbacephams induced by titanocene(III) chloride. J. Org. Chem. 2003, 68, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Walczak, K.; Dolg, M.; Piestert, F.; Lauterbach, T.; Worgull, D.; Gansäuer, A. Titanocene catalyzed 4-exo cyclizations: Mechanism, experiment, catalyst design. J. Am. Chem. Soc. 2008, 130, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Worgull, D.; Knebel, K.; Huth, I.; Schnakenburg, G. 4-Exo cyclizations by template catalysis. Angew. Chem. Int. Ed. 2009, 48, 8882–8885. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Greb, A.; Huth, I.; Worgull, D.; Knebel, K. Formal total synthesis of (+/-)-fragranol via template catalyzed 4-exo cyclization. Tetrahedron 2009, 65, 10791–10796. [Google Scholar] [CrossRef]
- Gansäuer, A.; Knebel, K.; Kube, C.; van Gastel, M.; Cangonul, A.; Daasbjerg, K.; Hangele, T.; Hulsen, M.; Dolg, M.; Friedrich, J. Radical 4-exo cyclization via template catalysis. Chem. Eur. J. 2012, 18, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, F.E.; Sarpong, M.A. Radical cyclization studies directed toward the synthesis of BMS-200475 ‘entecavir’: The carbocyclic core. Tetrahedron 2003, 59, 9013–9018. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; Herrero Teijón, P.; Rabanes Clemente, R.; Rubio Gonzalez, R. Radical reactions of epoxy esters induced by titanocene chloride. Tetrahedron Lett. 2006, 47, 7755–7758. [Google Scholar] [CrossRef]
- Leca, D.; Fensterbank, L.; Lacôte, E.; Malacria, M. Titanium-mediated domino radical cyclization/beta elimination of phosphine oxides. Angew Chem. Int. Ed. 2004, 43, 4220–4222. [Google Scholar] [CrossRef] [PubMed]
- Leca, D.; Song, K.; Albert, M.; Goançalves, M.G.; Fensterbank, L.; Lacôte, E.; Malacria, M. Titanocene-mediated intramolecular radical vinylations. Synthesis 2005, 1405–1420. [Google Scholar] [CrossRef]
- Gansäuer, A.; Rinker, B.; Pierobon, M.; Grimme, S.; Gerenkamp, M.; Muck-Lichtenfeld, C. A radical tandem reaction with homolytic cleavage of a Ti-O bond. Angew. Chem. Int. Ed. 2003, 42, 3687–3690. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Rinker, B.; Ndene-Schiffer, N.; Pierobon, M.; Grimme, S.; Gerenkamp, M.; Muck-Lichtenfeld, C. A radical roundabout for an unprecedented tandem reaction including a homolytic substitution with a titanium-oxygen bond. Eur. J. Org. Chem. 2004, 2337–2351. [Google Scholar] [CrossRef]
- Gansäuer, A.; Fleckhaus, A.; Lafont, M.A.; Okkel, A.; Kotsis, K.; Anoop, A.; Neese, F. Catalysis via homolytic substitutions with C-O and Ti-O bonds: Oxidative additions and reductive eliminations in single electron steps. J. Am. Chem. Soc. 2009, 131, 16989–16999. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.; Radetich, B.; Shin, S.; RajanBabu, T.V. Silylstannylation of highly functionalized acetylenes. Synthesis of precursors for annulations via radical or Heck reactions. Org. Lett. 2004, 6, 4053–4056. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Pierobon, M.; Bluhm, H. Stereoselective synthesis of tri- and tetrasubstituted olefins by tandem cyclization addition reactions featuring vinyl radicals. Angew. Chem. Int. Ed. 2002, 41, 3206–3208. [Google Scholar] [CrossRef]
- Trost, B.M.; Shen, H.C.; Surivet, J.P. An enantioselective biomimetic total synthesis of (−)-siccanin. Angew. Chem. Int. Ed. 2003, 42, 3943–3947. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Shen, H.C.; Surivet, J.P. Biomimetic enantioselective total synthesis of (-)-siccanin via the Pd-catalyzed asymmetric allylic alkylation (AAA) and sequential radical cyclizations. J. Am. Chem. Soc. 2004, 126, 12565–12579. [Google Scholar] [CrossRef] [PubMed]
- Haruo, Y.; Hasegawa, T.; Tanaka, H.; Takahashi, T. Total synthesis of (+/-)-smenospondiol by titanium(III)-mediated tandem radical cyclization. Synlett 2001, 1935–1937. [Google Scholar] [CrossRef]
- Arteaga, J.F.; Diéguez, H.R.; González-Delgado, J.A.; Quílez del Moral, J.F.; Barrero, A.F. Control of the regio- and diastereoselectivity for the preparation of highly functionalized terpenic cyclopentanes through radical cyclization. Eur. J. Org. Chem. 2011, 5002–5011. [Google Scholar] [CrossRef]
- Saha, S.; Roy, S.C. Titanocene (III) chloride mediated radical induced synthesis of (-)-methylenolactocin and (-)-protolichesterinic acid. Tetrahedron 2010, 66, 4278–4283. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; Ramos Silvo, A.I.; González, R.R.; Simmonds, M.S.J. Synthesis of CDE molecular fragments related to sendanin mediated by titanocene(III). Org. Biomol. Chem. 2012, 10, 5620–5628. [Google Scholar] [CrossRef] [PubMed]
- Cuerva, J.M.; Justicia, J.; Oller-López, J.L.; Bazdi, B.; Oltra, J.E. The growing impact of titanocene(III)-mediated radical epoxide opening on the synthesis of natural products. Mini-Rev. Org. Chem. 2006, 3, 23–35. [Google Scholar] [CrossRef]
- Gansäuer, A.; Justicia, J.; Rosales, A.; Worgull, D.; Rinker, B.; Cuerva, J.M.; Oltra, J.E. Transition-metal-catalyzed allylic substitution and titanocene-catalyzed epoxypolyene cyclization as a powerful tool for the preparation of terpenoids. Eur. J. Org. Chem. 2006, 4115–4127. [Google Scholar] [CrossRef]
- Yamaoka, M.; Fukatsu, Y.; Nakazaki, A.; Kobayashi, S. Synthetic study of fomitellic acids: Construction of the AB ring moiety. Tetrahedron Lett. 2009, 50, 3849–3852. [Google Scholar] [CrossRef]
- Justicia, J.; Oller-López, J.L.; Campaña, A.G.; Oltra, J.E.; Cuerva, J.M.; Buñuel, E.; Cárdenas, D.J. 7-Endo radical cyclizations catalyzed by titanocene (III). Straightforward synthesis of terpenoids with seven-membered carbocycles. J. Am. Chem. Soc. 2005, 127, 14911–14921. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Roy, S.C. Titanocene(III) mediated 8-endo radical cyclizations for the synthesis of eight-membered cyclic ethers. Tetrahedron Lett. 2006, 47, 1599–1601. [Google Scholar] [CrossRef]
- di Lena, F.; Matyjaszewski, K. Transition metal catalysts for controlled radical polymerization. Prog. Polym. Sci. 2010, 35, 959–1021. [Google Scholar] [CrossRef]
- Nomura, K. New approaches in precise synthesis of polyolefins containing polar functionalities by olefin copolymerizations using transition metal catalysis. J. Synth. Org. Chem. Jpn. 2012, 68, 1150–1158. [Google Scholar] [CrossRef]
- Kwark, Y.-J.; Kim, J.; Novak, B.M. Titanium complexes: A possible catalyst for controlled radical polymerization. Macromol. Res. 2007, 15, 31–38. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Patra, B.N. [CP2TiCl2] catalyzed polymerization in water: polymerization of methylmethacrylate to a high molecular weight polymer. Polymer 2004, 45, 3111–3114. [Google Scholar] [CrossRef]
- Asandei, A.D.; Moran, I.W. TiCp2Cl-catalyzed living radical polymerization of styrene initiated by oxirane radical ring opening. J. Am. Chem. Soc. 2004, 126, 15932–15933. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, W.; Sheng, Y.; Huang, Q.; Yang, W. Synthesis of well-defined star-shaped organosiloxane-functionalized polymethylmethacrylate promoted by epoxide-derived titanocene alkoxides via radical polymerization. J. Appl. Polym. Sci. 2011, 12, 1652–1658. [Google Scholar] [CrossRef]
- Asandei, A.D.; Saha, G. Living ring-opening polymerization of cyclic esters with epoxide-derived titanium alkoxides. Macromol. Rapid. Commun. 2005, 26, 626–631. [Google Scholar] [CrossRef]
- Asandei, A.D.; Simpson, C.P.; Yu, H.S. Cp2TiCl-catalyzed controlled radical polymerization of isoprene initiated from epoxides, aldehydes and halides. Polym. Prepr. Am. Chem. Soc. 2008, 49, 73–74. [Google Scholar]
- Asandei, A.D.; Simpson, C.P. Cp2TiCl-catalyzed synthesis of styrene/isoprene copolymers by controlled radical polymerization. Polym. Prepr. Am. Chem. Soc. 2008, 49, 75–76. [Google Scholar]
- Asandei, A.D.; Moran, I.W. The ligand effect in Ti-mediated living radical styrene polymerizations initiated by epoxide radical ring opening. I. Alkoxide and bisketonate Ti complexes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6028–6038. [Google Scholar] [CrossRef]
- Asandei, A.D.; Moran, I.W. The ligand effect in Ti-mediated living radical styrene polymerizations initiated by epoxide radical ring opening. II. Scorpionate and half-sandwich LTiCl3 complexes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6039–6047. [Google Scholar] [CrossRef]
- Asandei, A.D.; Moran, I.W. The ligand effect in Ti-mediated living radical styrene polymerizations initiated by epoxide radical ring opening. III. Substituted sandwich metallocenes. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1060–1070. [Google Scholar] [CrossRef]
- Asandei, A.D.; Moran, I.W.; Saha, G.; Chen, Y. Titanium-mediated living radical styrene polymerizations. V. Cp2TiCl-catalyzed initiation by epoxide radical ring opening: Effect of solvents and additives. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2015–2026. [Google Scholar] [CrossRef]
- Asandei, A.D.; Moran, I.W.; Saha, G.; Chen, Y. Titanium-mediated living radical styrene polymerizations. VI. Cp2TiCl-catalyzed initiation by epoxide radical ring opening: effect of the reducing agents, temperature, and titanium/epoxide and titanium/zinc ratios. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2156–2165. [Google Scholar] [CrossRef]
- Asandei, A.D.; Chen, Y. Cp2TiCl-catalyzed set reduction of aldehydes: A new initiating protocol for living radical polymerization. Macromolecules 2006, 39, 7549–7554. [Google Scholar] [CrossRef]
- Asandei, A.D.; Chen, Y.; Adebolu, O.I.; Simpson, C.P. Living ring-opening polymerization of ɛ-caprolactone with Ti alkoxides derived from the Cp2TiCl-catalyzed SET reduction of aldehydes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2869–2877. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevèe, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. On the use of bis(cyclopentadienyl)titanium(IV) dichloride in visible-light-induced ring-opening photopolymerization. Macromolecules 2012, 45, 356–361. [Google Scholar] [CrossRef]


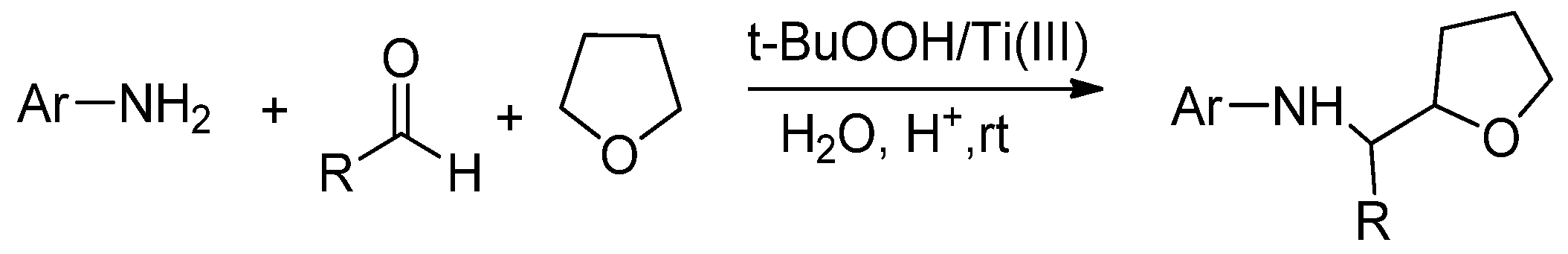


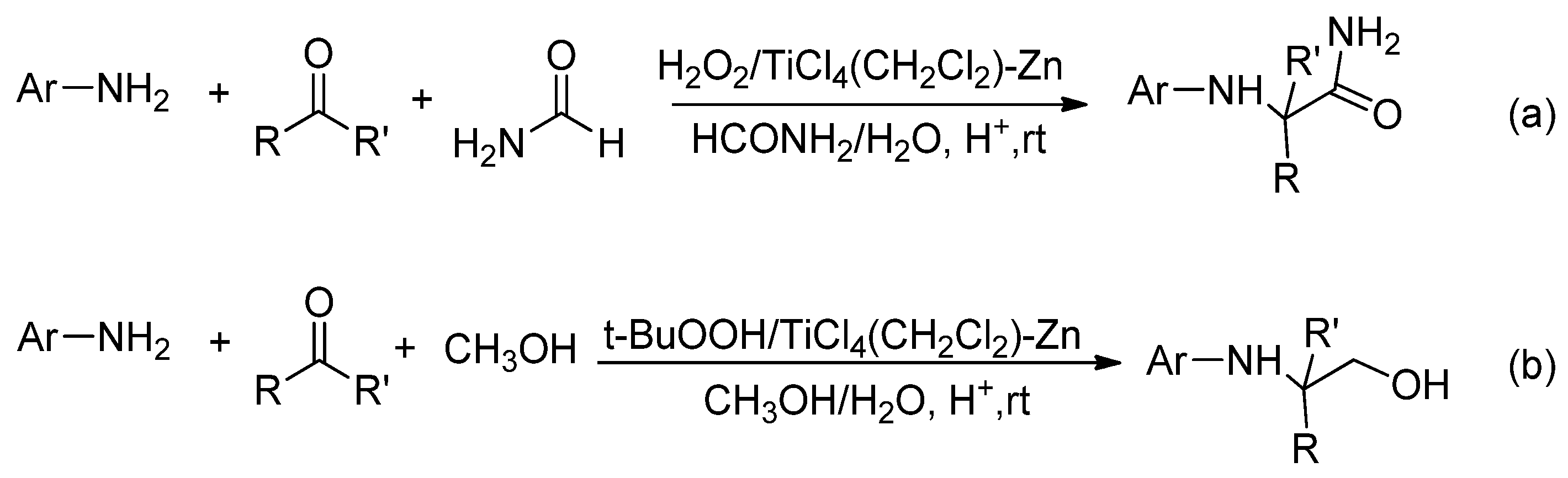



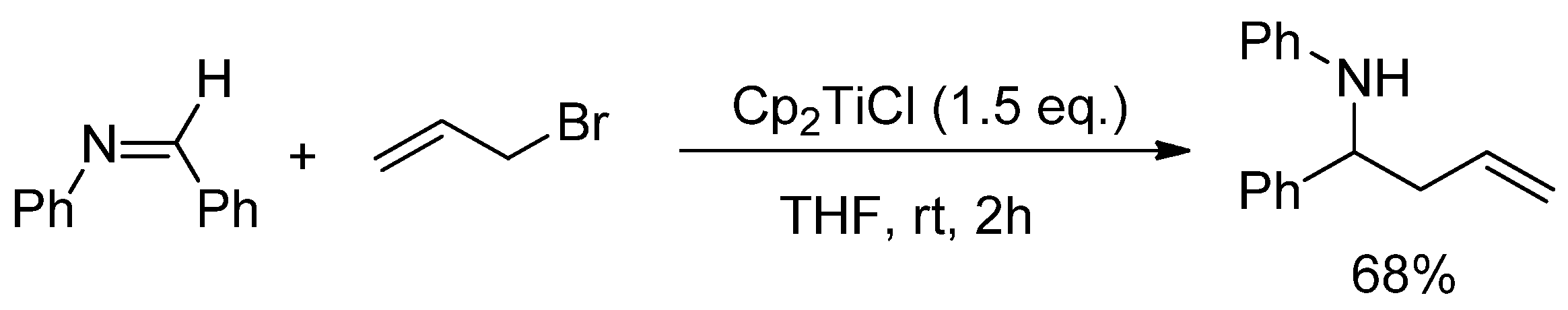





























© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rossi, B.; Prosperini, S.; Pastori, N.; Clerici, A.; Punta, C. New Advances in Titanium-Mediated Free Radical Reactions. Molecules 2012, 17, 14700-14732. https://doi.org/10.3390/molecules171214700
Rossi B, Prosperini S, Pastori N, Clerici A, Punta C. New Advances in Titanium-Mediated Free Radical Reactions. Molecules. 2012; 17(12):14700-14732. https://doi.org/10.3390/molecules171214700
Chicago/Turabian StyleRossi, Bianca, Simona Prosperini, Nadia Pastori, Angelo Clerici, and Carlo Punta. 2012. "New Advances in Titanium-Mediated Free Radical Reactions" Molecules 17, no. 12: 14700-14732. https://doi.org/10.3390/molecules171214700




