Study of Rigid Cross-Linked PVC Foams with Heat Resistance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gel Content Measurement
2.2. Thermal Mechanical Analysis (TMA)
2.3. Differential Scanning Calorimetry (DSC) Analysis
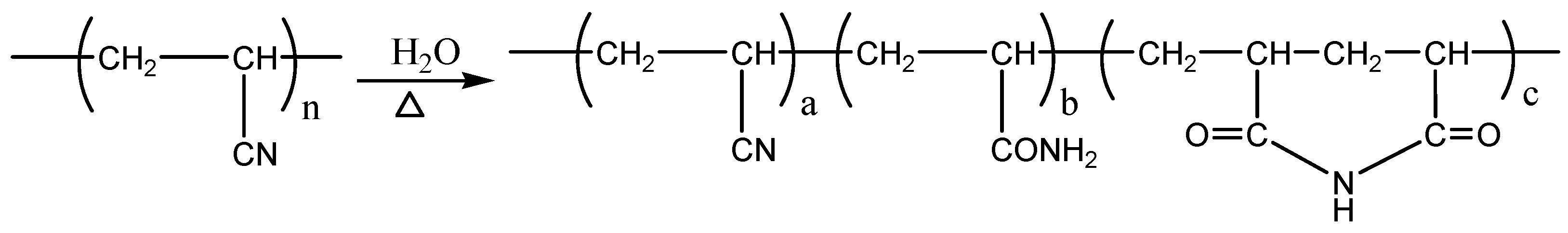
2.4. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG) Analysis
2.5. Heat Distortion Temperature (HDT) Analysis
2.6. Dimension Stability Analysis
2.7. Mechanical Properties Analysis
3. Experimental
3.1. Materials
3.2. Experiment Preparation
3.3. Characterization
3.3.1. Gel Content Measurement

3.3.2. Heat Resistance Analysis
3.3.3. Mechanical Property Analysis
4. Conclusions
Acknowledgments
References
- Shi, A.H.; Zhang, G.C.; Pan, H.T.; Ma, Z.L.; Zhao, C.H. The preparation and properties of rigid cross-linked PVC foam. Adv. Mater. Res. 2011, 311–313, 1056–1060. [Google Scholar] [CrossRef]
- Heider, D.; Simacek, P.; Dominauskas, A.; Deffor, H.; Advani, S.; Gillespie, J.W., Jr. Infusion design methodology for thick-section, low-permeability preforms using inter-laminar flow media. Compos. Part A Appl. Sci. Manuf. 2007, 38, 525–534. [Google Scholar] [CrossRef]
- Zhao, C.H.; Zhang, G.C.; Wu, Y.B. Resin flow behavior simulation of grooved foam sandwich composites with the vacuum assisted resin infusion (VARI) molding process. Materials 2012, 5, 1285–1296. [Google Scholar] [CrossRef]
- Grujicic, M.; Chittajallu, K.M.; Walsh, S. Optimization of the VARTM process for enhancement of the degree of devolatilization of polymerization by-products and solvents. J. Mater. Sci. 2003, 38, 3729–3739. [Google Scholar] [CrossRef]
- Lin, H.R. The structure and property relationships of commercial foamed plastics. Polym. Test. 1997, 16, 429–443. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.J.; Zhang, J. Improvement in the heat resistance of poly(vinyl chloride) profile with styrenic polymers. J. Vinyl Addit. Technol. 2011, 17, 85–91. [Google Scholar] [CrossRef]
- Zadhoush, A.; Esmaeili, M.; Ghaeli, I. Crosslinking of plasticized PVC used in coated fabrics. J. Vinyl Addit. Technol. 2009, 15, 108–112. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.S.; Shao, L.; Zhao, J.R.; Feng, Y. Structure and properties of chlorinated polyvinyl chloride graft copolymer with higher property. Polym. Adv. Technol. 2012, 23, 470–477. [Google Scholar] [CrossRef]
- Kelkar, D.S.; Soman, V.V. Study of structural, morphological and mechanical properties of PMMA, PVC and their blends. Radiat. Eff. Defect. Solid. 2012, 167, 120–130. [Google Scholar] [CrossRef]
- Singh, A.; Rawat, M.S.M.; Pande, C.S. Chemical modification and characterization of poly(vinyl chloride) by crosslinking of multifunctional amines. J. Appl. Polym. Sci. 2010, 118, 876–880. [Google Scholar] [CrossRef]
- Gunewardena, A.; Gilbert, M. Peroxide crosslinking of rigid poly(vinyl chloride). J. Vinyl Addit. Technol. 2008, 14, 92–98. [Google Scholar] [CrossRef]
- Thomas, N.L.; Zheng, X. Peroxide crosslinking of rigid poly(vinyl chloride). J. Appl. Polym. Sci. 2007, 103, 2904–2909. [Google Scholar] [CrossRef]
- Saethre, B.; Gilbert, M. Peroxide crosslinking of plasticized poly(vinyl chloride). Polymer 1996, 37, 3379–3386. [Google Scholar] [CrossRef]
- Fiaz, M.; Gilbert, M. Silane crosslinking of plasticized poly(vinyl chloride). Adv. Polym. Technol. 1998, 17, 37–51. [Google Scholar] [CrossRef]
- Hearn, M.S.; Baird, J.D.; Nethsinghe, L.P.; Gilbert, M. Silane cross-linking of plasticized poly(vinyl chloride). Polym. Commun. 1990, 31, 194–197. [Google Scholar]
- Yanez-Flores, I.G.; Ibarra-Gomez, R.; Rodriguez-Fernandez, O.S.; Gilbert, M. Peroxide crosslinking of PVC foam formulations. Eur. Polym. J. 2000, 36, 2235–2241. [Google Scholar] [CrossRef]
- Danielsson, M.; Grenestedt, J.L. Gradient foam core materials for sandwich structures: Preparation and characterisation. Compos. Part A Appl. Sci. Manuf. 1998, 29, 981–988. [Google Scholar] [CrossRef]
- Reyes-Labarta, J.A.; Marcilla, A. Thermal treatment and degradation of cross-linked ethylene vinyl acetate-polyethylene-azodicarbonamide-ZnO foams. Complete kinetic modeling and analysis. Ind. Eng. Chem. Res. 2012, 51, 9515–9530. [Google Scholar] [CrossRef]
- Reyes-Labarta, J.A.; Marcilla, A. Kinetic study of the decompositions involved in the thermal degradation of commercial azodicarbonamide. J. Appl. Polym. Sci. 2008, 107, 339–346. [Google Scholar] [CrossRef]
- Garcia-Quesada, J.C.; Gilbert, M. Peroxide crosslinking of unplasticized poly(vinyl chloride). J. Appl. Polym. Sci. 2000, 77, 2657–2666. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |






| Foam types | Gel content/% | |
|---|---|---|
| Molded products | Foam plastics | |
| universal cross-linked PVC structural foam | 0 | 49.3 |
| heat resistant cross-linked PVC foam I | 30.6 | 80.1 |
| heat resistant cross-linked PVC foam II | 35.5 | 86.3 |
| heat resistant cross-linked PVC foam III | 0 | 96.4 |
| Foam type | Td5/°C | Td10/°C | Td/°C | Td50/°C | Residual weight/% |
|---|---|---|---|---|---|
| universal cross-linked PVC structural foam | 172 | 206 | 257 | 331 | 18.08 |
| heat resistant cross-linked PVC foam I | 189 | 245 | 259 | 339 | 21.41 |
| heat resistant cross-linked PVC foam II | 189 | 250 | 259 | 349 | 22.38 |
| heat resistant cross-linked PVC foam III | 252 | 267 | 265 | 357 | 23.38 |
| Temperature | 80 °C | 100 °C | 120 °C | 140 °C | 160 °C a | |
|---|---|---|---|---|---|---|
| Weight change rate/% | heat resistant foam I | −0.95 | −1.73 | −2.36 | −4.81 | −7.20 |
| heat resistant foam II | −0.93 | −1.73 | −2.31 | −4.73 | −7.04 | |
| heat resistant foam III | −0.85 | −1.58 | −2.24 | −3.98 | −6.33 | |
| universal structural foam | −1.06 | −2.48 | −3.89 | −5.17 | −10.97 | |
| Volume change rate/% | heat resistant foam I | −2.85 | −4.48 | −5.74 | −2.03 | +28.41 |
| heat resistant foam II | −2.73 | −4.37 | −5.39 | −1.04 | +32.27 | |
| heat resistant foam III | −2.03 | −3.52 | −5.04 | +1.16 | +34.06 | |
| universal structural foam | −3.41 | −8.51 | −11.23 | −5.77 | +15.64 | |
| Foam plastic | I | II | III | Universal |
|---|---|---|---|---|
| Compressive strength /MPa | 1.17 | 1.23 | 1.21 | 1.11 |
| Compressive modulus/Mpa | 51.5 | 52.9 | 52.1 | 49.4 |
| Tensile strength /Mpa | 1.77 | 1.76 | 1.73 | 1.69 |
| Tensile modulus /Mpa | 57.3 | 58.2 | 57.0 | 55.8 |
| Elongation at break /% | 9.1 | 9.0 | 5.5 | 8.4 |
| Shear strength /Mpa | 1.01 | 0.99 | 0.92 | 0.95 |
| Shear modulus /Mpa | 29.8 | 30.2 | 29.8 | 28.4 |
| Formulation | Universal | I | II | III |
|---|---|---|---|---|
| PVC | 100 | 100 | 100 | 100 |
| MDI-L | 64 | 64 | 64 | 64 |
| MHHPA | 29 | 29 | 29 | - |
| AIBN | 3 | 3 | 3 | 3 |
| AC | 1 | 1 | 1 | 1 |
| ESO | 3 | 3 | 3 | 3 |
| DCP | - | 1 | 1 | 1 |
| TMPTMA | - | 5 | - | - |
| TAIC | - | - | 10 | - |
| MAH | - | - | - | 17 |
| AN | - | - | - | 4 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, A.; Zhang, G.; Zhao, C. Study of Rigid Cross-Linked PVC Foams with Heat Resistance. Molecules 2012, 17, 14858-14869. https://doi.org/10.3390/molecules171214858
Shi A, Zhang G, Zhao C. Study of Rigid Cross-Linked PVC Foams with Heat Resistance. Molecules. 2012; 17(12):14858-14869. https://doi.org/10.3390/molecules171214858
Chicago/Turabian StyleShi, Aihua, Guangcheng Zhang, and Chenhui Zhao. 2012. "Study of Rigid Cross-Linked PVC Foams with Heat Resistance" Molecules 17, no. 12: 14858-14869. https://doi.org/10.3390/molecules171214858




