Structural Changes of Oil Palm Empty Fruit Bunch (OPEFB) after Fungal and Phosphoric Acid Pretreatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Pretreatment on Biomass Components
| ASL (%) | AIL (%) | Total Lignin (%) | Cellulose (%) | Hemicellulose (%) | Total Solid Loss (%) | |
|---|---|---|---|---|---|---|
| Untreated OPEFB | 7.81 ± 0.03 | 26.56 ± 0.14 | 34.37 ± 0.17 | 39.13 ± 2.26 | 23.04 ± 2.79 | 0 |
| Fungal pretreatment | 8.39 ± 0.40 | 25.95 ± 0.36 | 34.34 ± 0.36 | 34.17 ± 0.40 | 27.48 ± 9.07 | 1.31 ± 0.13 |
| Phosphoric acid pretreatment | 4.30 ± 0.09 | 40.13 ± 0.51 | 44.66 ± 0.18 | 43.16 ± 0.43 | 9.07 ± 0.05 | 54.84 ± 1.37 |
| Fungal followed by phosphoric acid pretreatment | 4.53 ± 0.05 | 32.92 ± 0.60 | 37.22 ± 0.51 | 53.81 ± 1.14 | 9.07 ± 0.14 | 63.55 ± 0.76 |
| ASL (%) | AIL (%) | Total Lignin (%) | Cellulose (%) | Hemicellulose (%) | Total Carbohydrate (%) | |
|---|---|---|---|---|---|---|
| Untreated OPEFB | 0 | 0 | 0 | 0 | 0 | 0 |
| Fungal pretreatment | −0.47 * | 0.95 | 0.48 | 5.40 | 2.48 | 7.88 |
| Phosphoric acid pretreatment | 5.51 | 6.19 | 11.70 | 17.22 | 18.43 | 35.65 |
| Fungal following phosphoric acid pretreatment | 5.87 | 11.69 | 17.56 | 14.83 | 18.94 | 33.77 |

2.2. Effects of Pretreatment on the OPEFB Structure

| Untreated OPEFB | Fungal pretreatment | Phosphoric acid pretreatment | Fungal followed by phosphoric acid pretreatment | Assignments | Source | Ref. |
|---|---|---|---|---|---|---|
| Wavenumber (cm−1) | ||||||
| 648 | 666 | 666 | 667 | C-O out-of-plane bending mode | Cellulose | [29] |
| 716 | - | - | - | Rocking vibration CH2 in Cellulose Iβ | Cellulose | [29] |
| 770 | 770 | 769 | 769 | CH2 vibration in Cellulose Iα | Cellulose | [29] |
| 849 | 851 | 850 | 851 | C-H out of plane deformation in position 2,5,6 | G-Lignin | [30] |
| 897 | 896 | 895 | 895 | Anomere C-groups C(1)-H deformation, ring valence vibration | Polysaccharides | [30,31] |
| - | - | 998 | 997 | C-O valence vibration | [29] | |
| 1,032 | 1,033 | 1,022 | 1,022 | Aromatic C-H in plane deformation, G > S; plus C-O deformation in primary alcohols; plus C=O stretch (unconj.) | Lignin | [29] |
| 1,159 | 1,159 | 1,158 | 1,158 | C-O-C assimetric valence vibration | Polysaccharides | [30] |
| - | - | 1,224 | 1,223 | C-C plus C-O plus C=O strech; G condensed > G etherified | Polysaccharides | [30,31] |
| 1,241 | 1,237 | 1,243 | 1,245 | C=O stretch, OH i.p. bending | [32] | |
| 1,266 | 1,267 | 1,267 | 1,267 | G-ring plus C=O strectch | G-Lignin | [33] |
| 1,321 | 1,326 | 1,315 | 1,315 | O-H blending of alcohol groups | Carbohydrate | [30] |
| 1,375 | 1,371 | 1,370 | 1,372 | C-H deformation vibration | Cellulose | [31] |
| 1,418 | 1,418 | 1,420 | 1,419 | Aromatic skeletal vibrations with C-H in plane deformation CH2 scissoring | Lignin | [34] |
| 1,462 | 1,457 | 1,455 | 1,459 | C-H in pyran ring symmetric scissoring; OH plane deformation vibration | Cellulose | [31] |
| 1,511 | 1,507 | 1,506 | 1,506 | Aromatic skeletal vibrations;G > S | Lignin | [34] |
| 1,593 | 1,609 | 1,608 | 1,607 | Aromatic skeletal vibrations plus C=O stretch; S > G; G condensed > G etherified | Lignin | [34] |
| 1,640 | 1,646 | 1,654 | 1,663 | C O stretch in conjugated p-substituted aryl ketones | Lignin | [34] |
| 1,735 | 1,735 | 1,735 | 1,735 | CO stretch unconjugated (xylan) | Polysaccharides | [34] |
| 2,850 | 2,850 | 2,850 | 2,850 | Asymetric CH2 valence vibration | [29] | |
| 2,918 | 2,918 | 2,918 | 2,918 | Symmetric CH2 valence vibration | [29] | |
| 3,338 | 3,345 | 3,346 | 3,351 | Hydrogen bonded O-H valence vibration; O(3)H...O(3) intermolecular in cellulose | Cellulose | [29] |

2.3. Effect of Pretreatments on OPEFB Morphology
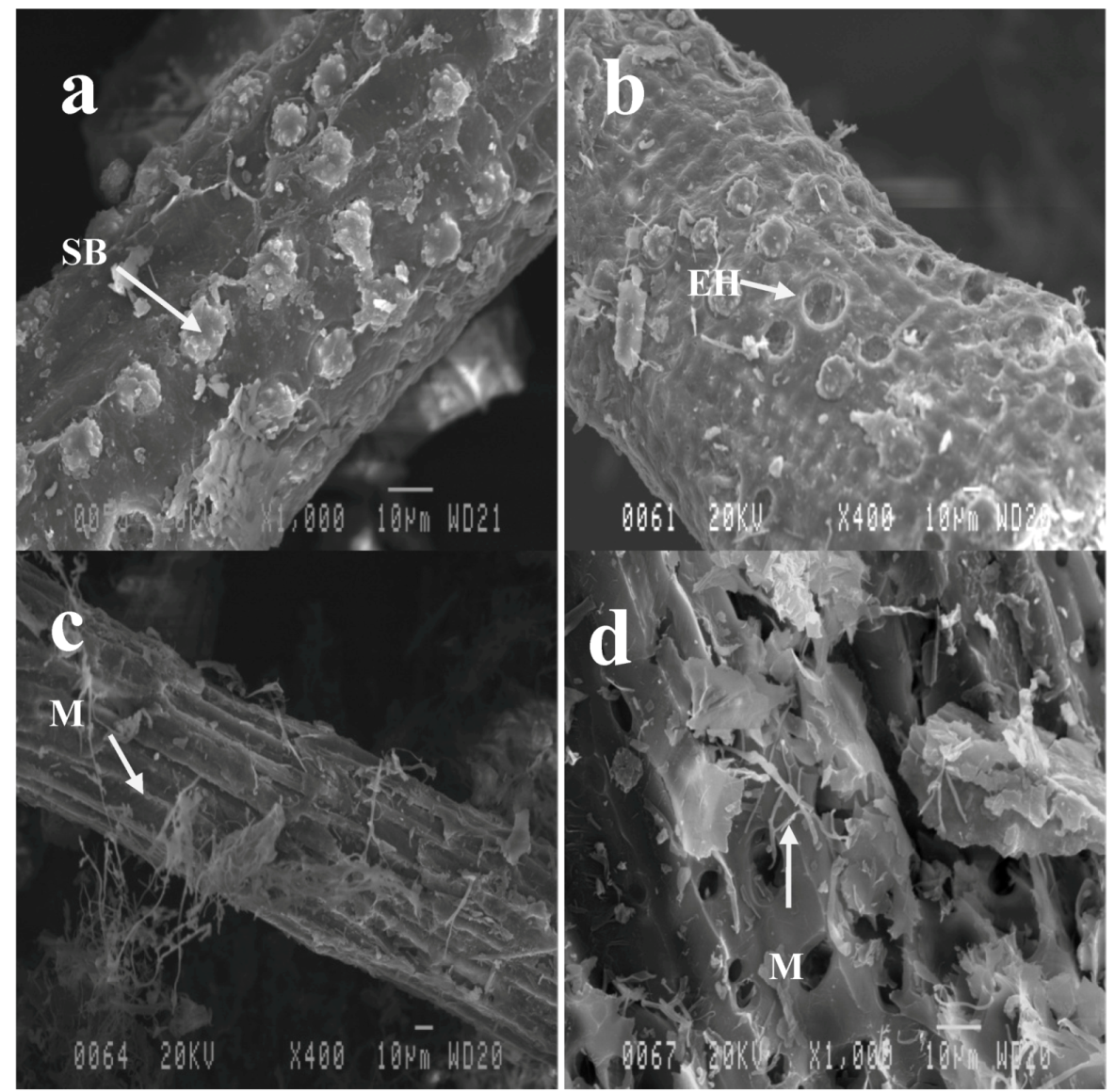

2.4. Cellulose Digestibility of Untreated and Pretreated OPEFB

3. Experimental
3.1. Oil Palm Empty Fruit Bunch
3.2. Microorganism
3.3. Biological Pretreatment
3.4. Phosphoric Acid Pretreatment
3.5. Enzymatic Hydrolysis

3.6. Analytical Methods
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the OPEFB are available from the authors.
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Oli palm fruits, area harvested:. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor/ (accessed on 28 October 2012).
- Law, K.N.; Daud, W.R.W.; Ghazali, A. Morphological and chemical nature of fiber strands of oil palm empty-fruit-bunch (OPEFB). BioResources 2007, 2, 351–362. [Google Scholar]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Piarpuzán, D.; Quintero, J.A.; Cardona, C.A. Empty fruit bunches from oil palm as a potential raw material for fuel ethanol production. Biomass Bioenergy 2011, 35, 1130–1137. [Google Scholar] [CrossRef]
- Scott, G.M.; Akhtar, M.; Swaney, R.E.; Houtman, C.J. Recent developments in biopulping technology at Madison, WI. Prog. Biotechnol. 2002, 21, 61–71. [Google Scholar] [CrossRef]
- Okano, K.; Ohkoshi, N.; Nishiyama, A.; Usagawa, T.; Kitagawa, M. Improving the nutritive value of madake bamboo, Phyllostachys bambusoides, for ruminants by culturing with the white-rot fungus Ceriporiopsis subvermispora. Anim. Feed Sci. Technol. 2009, 152, 278–285. [Google Scholar] [CrossRef]
- Hölker, U.; Hölker, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef]
- Rivers, D.B.; Emert, G.H. Factors affecting the enzymatic hydrolysis of bagasse and rice straw. Biol. Wastes 1988, 26, 85–95. [Google Scholar] [CrossRef]
- Anderson, W.F.; Akin, D.E. Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. J. Ind. Microbiol. Biotechnol. 2008, 35, 355–366. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Zhu, L.; O’Dwyer, J.P.; Chang, V.S.; Granda, C.B.; Holtzapple, M.T. Structural features affecting biomass enzymatic digestibility. Bioresour. Technol. 2008, 99, 3817–3828. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Waste to Improve Ethanol and Biogas Production. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Hatakka, A.I. Pretreatment of wheat straw by white-rot fungi for enzymatic saccharification of cellulose. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 350–357. [Google Scholar]
- Taniguchi, M.; Suzuki, H.; Watanabe, D.; Sakai, K.; Hoshino, K.; Tanaka, T. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 2005, 100, 637–643. [Google Scholar] [CrossRef]
- Ray, M.J.; Leak, D.J.; Spanu, P.D.; Murphy, R.J. Brown rot fungal early stage decay mechanism as a biological pretreatment for softwood biomass in biofuel production. Biomass Bioenergy 2010, 34, 1257–1262. [Google Scholar] [CrossRef]
- Kurakake, M.; Ide, N.; Komaki, T. Biological Pretreatment with Two Bacterial Strains for Enzymatic Hydrolysis of Office Paper. Curr. Microbiol. 2007, 54, 424–428. [Google Scholar]
- Kirk, T.K.; Chang, H.-M. Potential applications of bio-ligninolytic systems. Enzyme Microb. Technol. 1981, 3, 189–196. [Google Scholar] [CrossRef]
- Zadražil, F.; Puniya, A.K. Studies on the effect of particle size on solid-state fermentation of sugarcane bagasse into animal feed using white-rot fungi. Bioresour.Technol. 1995, 54, 85–87. [Google Scholar] [CrossRef]
- Martínez, A.T.; Camarero, S.; Guillén, F.; Gutiérrez, A.; Muñoz, C.; Varela, E.; Martínez, M.J.; Barrasa, J.; Ruel, K.; Pelayo, J. Progress in biopulping of non-woody materials: Chemical, enzymatic and ultrastructural aspects of wheat straw delignification with ligninolytic fungi from the genus Pleurotus. FEMS Microbiol. Rev. 1994, 13, 265–273. [Google Scholar]
- Isroi; Millati, R.; Syamsiah, S.; Niklasson, C.; Cahyanto, M.N.; Lundquist, K.; Taherzadeh, M.J. Biological pretreatment of lignocelluloses with white-rot fungi and its applications: A review. BioResources 2011, 6, 5224–5259. [Google Scholar]
- Taniguchi, M.; Takahashi, D.; Watanabe, D.; Sakai, K.; Hoshino, K.; Kouya, T.; Tanaka, T. Effect of steam explosion pretreatment on treatment with Pleurotus ostreatus for the enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 2010, 110, 449–452. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, L.; Ke, J.; Xu, C.; Du, W.; Zhang, J. Evaluation of white-rot fungi-assisted alkaline/oxidative pretreatment of corn straw undergoing enzymatic hydrolysis by cellulase. J. Biosci. Bioeng. 2010, 110, 660–664. [Google Scholar] [CrossRef]
- Itoh, H.; Wada, M.; Honda, Y.; Kuwahara, M.; Watanabe, T. Bioorganosolve pretreatments for simultaneous saccharification and fermentation of beech wood by ethanolysis and white rot fungi. J. Biotechnol. 2003, 103, 273–280. [Google Scholar]
- Ma, F.; Yang, N.; Xu, C.; Yu, H.; Wu, J.; Zhang, X. Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresour.Technol. 2010, 101, 9600–9604. [Google Scholar]
- Zhang, Y.-H.P.; Ding, S.-Y.; Mielenz, J.R.; Cui, J.-B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef]
- Nieves, D.C.; Karimi, K.; Horváth, I.S. Improvement of biogas production from oil palm empty fruit bunches (OPEFB). Ind. Crops Prod. 2011, 34, 1097–1101. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. Localisation and characterisation of incipient brown-rot decay within spruce wood cell walls using FT-IR imaging microscopy. Enzyme Microb. Technol. 2010, 47, 257–267. [Google Scholar] [CrossRef]
- Fengel, D. Characterization of Cellulose by Deconvoluting the OH Valency Range in FTIR Spectra. Holzforschung 1992, 46, 283–285. [Google Scholar] [CrossRef]
- Faix, O.; Böttcher, J.H. The influence of particle size and concentration in transmission and diffuse reflectance spectroscopy of wood. HolzAls Roh Werkst. 1992, 50, 221–226. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforchung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Faix, O.; Bremer, J.; Schmidt, O.; Tatjana, S.J. Monitoring of chemical changes in white-rot degraded beech wood by pyrolysis—Gas chromatography and Fourier-transform infrared spectroscopy. J. Anal. Appl. Pyrolysis. 1991, 21, 147–162. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Takahashi, N.; Koshijima, T. Ester linkages between lignin and glucuronoxylan in a lignin-carbohydrate complex from beech (Fagus crenata) wood. Wood Sci. Technol. 1988, 22, 231–241. [Google Scholar] [CrossRef]
- O’Sullivan, A. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Åkerholm, M.; Hinterstoisser, B.; Salmén, L. Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr. Res. 2004, 339, 569–578. [Google Scholar] [CrossRef]
- O’Connor, R.T.; DuPré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Hurtubise, F.G.; Krassig, H. Classification of Fine Structural Characteristics in Cellulose by Infared Spectroscopy. Use of Potassium Bromide Pellet Technique. Anal. Chem. 1960, 32, 177–181. [Google Scholar] [CrossRef]
- Evans, R.; Newman, R.; Roick, U. Changes in cellulose crystallinity during kraft pulping. Comparison of infrared, x-ray diffraction and solid state NMR results. Holzforschung 1995, 49, 498–504. [Google Scholar]
- Lau, M.; Gunawan, C.; Dale, B. Ammonia Fiber Expansion (AFEX) Pretreatment, Enzymatic Hydrolysis, and Fermentation on Empty Palm Fruit Bunch Fiber (EPFBF) for Cellulosic Ethanol Production. Appl. Biochem. Biotechnol. 2010, 162, 1847–1857. [Google Scholar] [CrossRef]
- Bahrin, E.K.; Baharuddin, A.S.; Ibrahim, M.F.; Razak, M.H.A.; Sulaiman, A.; Abd-Aziz, S.; Hassan, M.A.; Shirai, Y.; Nishida, H. Physicochemical property changed and enzymatic hydrolysis enhancement of oil palm emtpy fruit bunches treated with superheated steam. BioResources 2012, 7, 1784–1801. [Google Scholar]
- Hamzah, F.; Idris, A.; Shuan, T.K. Preliminary study on enzymatic hydrolysis of treated oil palm (Elaeis) empty fruit bunches fibre (EFB) by using combination of cellulase and β 1-4 glucosidase. Biomass Bioenergy 2011, 35, 1055–1059. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Enhancement of ethanol and biogas production from hight-crystalline cellulose by different modes of NMO pretreatment. Biotechnol. Bioeng. 2009, 105, 469–476. [Google Scholar]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2010, 108, 22–30. [Google Scholar]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass Technical Report No. NREL/TP-510-42629, 21 March 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass Technical Report No. NREL/TP-510-42618, 8 July 2011.
- Sluiter, A.D.; Hames, B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass Technical Report No. NREL/TP-510-42622, 2008.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Isroi; Ishola, M.M.; Millati, R.; Syamsiah, S.; Cahyanto, M.N.; Niklasson, C.; Taherzadeh, M.J. Structural Changes of Oil Palm Empty Fruit Bunch (OPEFB) after Fungal and Phosphoric Acid Pretreatment. Molecules 2012, 17, 14995-15012. https://doi.org/10.3390/molecules171214995
Isroi, Ishola MM, Millati R, Syamsiah S, Cahyanto MN, Niklasson C, Taherzadeh MJ. Structural Changes of Oil Palm Empty Fruit Bunch (OPEFB) after Fungal and Phosphoric Acid Pretreatment. Molecules. 2012; 17(12):14995-15012. https://doi.org/10.3390/molecules171214995
Chicago/Turabian StyleIsroi, Mofoluwake M. Ishola, Ria Millati, Siti Syamsiah, Muhammad N. Cahyanto, Claes Niklasson, and Mohammad J. Taherzadeh. 2012. "Structural Changes of Oil Palm Empty Fruit Bunch (OPEFB) after Fungal and Phosphoric Acid Pretreatment" Molecules 17, no. 12: 14995-15012. https://doi.org/10.3390/molecules171214995






