Supramolecular Photodimerization of Coumarins
Abstract
:1. Introduction


2. Supramolecular Photodimerization of Coumarins in Solution
2.1. Cucurbituril

2.2. Pd-Nanocages

3. Supramolecular Photodimerization of Coumarins in the Solid State
3.1. β-Cyclodextrin

3.2. Bisurea Macrocycle

3.3. Diacetylenediols

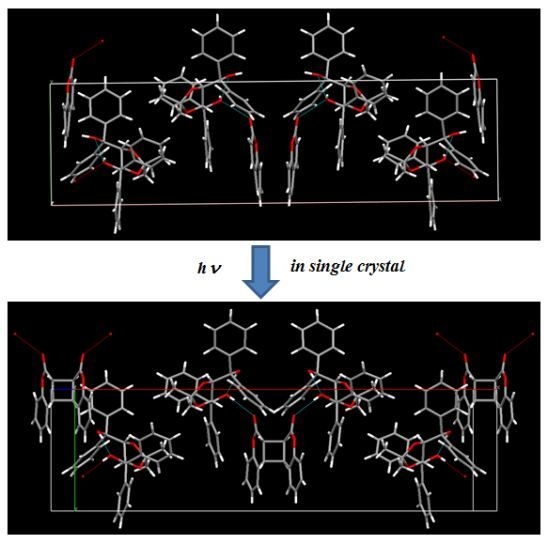
4. Enantioselective Photodimerization of Coumarins in Crystalline Inclusion Complexes




| Compound | (−)-23: 1 | (−)-23: (−)-2 | (−)-24: 25 | (−)-24: (+)-26 |
| Formula | C40H36O6 | C40H36O6 | C42H38O5S1 | C42H38O5S1 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2 | C2 | P21 | P21 |
| a (Å) | 35.59(4) | 32.80(3) | 10.235(2) | 10.371(3) |
| b (Å) | 9.489(4) | 9.467(3) | 35.78(1) | 34.70(2) |
| c (Å) | 10.03(1) | 10.36(4) | 9.422(2) | 9.414(3) |
| β (°) | 102.70(4) | 100.27(7) | 91.00(2) | 91.38(3) |
| V (Å3) | 3305(4) | 3164(2) | 3449(1) | 3387(2) |
| Z | 4 | 4 | 4 | 4 |
| Dcalc | 1.23 | 1.29 | 1.26 | 1.28 |
| R | 0.101 | 0.114 | 0.065 | 0.078 |
| Temperature (°C) | Room | Room | −50 | −50 |
5. Enantioselective Photodimerization of Coumarins in Solution


References
- Gnanaguru, K.; Ramasubbu, N.; Venkatesan, K.; Ramamurthy, V. A study on the photochemical dimerization of coumarins in the solid state. J. Org. Chem. 1985, 50, 2337–2346. [Google Scholar]
- Narasimha, J.; Venkatesan, K.; Weiss, R.G. Photodimerization of coumarins in solid cyclodextrin inclusion complexes. J. Org. Chem. 1992, 57, 3292–3297. [Google Scholar]
- Vishnumurthy, K.; Guru Row, T.N.; Venkatesan, K. Studies in crystal engineering: Effect of fluorine substitution in crystal packing and topological photodimerization of styryl coumarins in the solid state. J. Chem. Soc. Perkin Trans. 2 1996, 1475–1478. [Google Scholar]
- Yu, X.; Scheller, D.; Rademacher, O.; Wolff, T. Selectivity in the photodimerization of 6-alkylcoumarins. J. Org. Chem. 2003, 68, 7386–7399. [Google Scholar]
- Wolff, T.; Gorner, H. Photodimerization of coumarin revisited: Effects of solvent polarity on the triplet reactivity and product pattern. Phys. Chem. Chem. Phys. 2004, 6, 368–376. [Google Scholar]
- Mahon, M.F.; Raithby, P.R.; Sparkes, H.A. Investigation of the factors favoring solid state [2+2] cycloaddition reactions; the [2+2] cycloaddition reaction of coumarin-3-carboxylic acid. CrystEngComm 2008, 10, 573–576. [Google Scholar]
- Pemberton, B.C.; Barooah, N.; Srivatsava, D.K.; Sivaguru, J. Supramolecular photocatalysis by confinement-photodimerization of coumarins within cucurbit[8]urils. Chem. Commun. 2010, 46, 225–227. [Google Scholar]
- Karthikeyan, S.; Ramamurthy, V. Templating photodimerization of coumarins within a water-soluble nano reaction vessel. J. Org. Chem. 2006, 71, 6409–6413. [Google Scholar]
- Yu, X.; Scheller, D.; Rademacher, O.; Wolff, T. Photodimerization of coumarins in solid cyclodextrin complexes. J. Org. Chem. 1992, 57, 3292–3297. [Google Scholar]
- Brett, T.J.; Alexander, J.M.; Clark, J.L.; Ross, C.R., II.; Harbiso, G.S.; Stezowski, J.J. Chemical insight from crystallographic disorder: Structural studies of a supramolecular b-cyclodextrin/coumarin photochemical system. Chem. Commun. 1999, 1275–1276. [Google Scholar]
- Dawn, S.; Dewal, M.B.; Sobransingh, D.; Paderes, M.C.; Wibowo, A.C.; Smith, M.D.; Krause, J.A.; Pellechia, P.J.; Shimizu, L.S. Self-assembled phenylethynylene bis-urea macrocycles facilitate the selective photodimerization of coumarin. J. Am. Chem. Soc. 2011, 133, 7025–7032. [Google Scholar]
- Moorthy, J.N.; Venkatesan, K. Stereospecific photodimerization of coumarins in crystalline inclusion complexes. Molecular and crystal structure of 1:2 complex of (S,S)-(−)-1,6-bis(o-chlorophenyl)-1,6-diphenyl-hexa-2,4-diyne-1,6-diol and coumarin. J. Org. Chem. 1991, 56, 6957–6960. [Google Scholar]
- Tanaka, K.; Toda, F. Selective photodimerizations of coumarin in crystalline inclusion compounds. J. Chem. Soc. Perkin Trans. 1 1992, 943–944. [Google Scholar]
- Tanaka, K.; Toda, F.; Mochizuki, E.; Yasui, N.; Kai, Y.; Miyahara, I.; Hirotsu, K. Enantioselective single-crystal-to-single-crystal photodimerization of coumarin and thiocoumarin in inclusion complexes with chiral host compounds. Angew. Chem. Int. Ed. 1999, 38, 3523–3525. [Google Scholar]
- Tanaka, K.; Mochizuki, E.; Yasui, N.; Kai, Y.; Miyahara, I.; Hirotsu, K.; Toda, F. Single-crystal-to-single-crystal enantioselective [2+2] photodimerization of coumarin, thiocoumarin and cyclohex-2-enone in the inclusion complexes with chiral host compounds. Tetrahedron 2000, 56, 6853–6865. [Google Scholar]
- Zhao, D.; Sun, J.; Ding, K. New types of soluble polymer-supported bisphosphine ligands with a cyclobutane backbone for Pd-catalyzed enantioselective allylic substitution reactions. Chem. Eur. J. 2004, 10, 5952–5963. [Google Scholar]
- Klaus, C.P.; Thiemann, C.; Kopf, J.; Margaretha, P. Solid-state photocyclodimerization of 1-thiocoumarin. Helv. Chim. Acta 1995, 78, 1079–1082. [Google Scholar]
- Wen, Y.; Song, Y.; Zhao, D.; Ding, K.; Bian, J.; Zhang, X.; Wang, J.; Liu, Y.; Jianga, L.; Zhu, D. Highly regio- and enantioselective thermal [2+2] cycloaddition of coumarin in a crystalline inclusion complex under high vacuum. Chem. Commun. 2005, 2732–2734. [Google Scholar]
- Tanaka, K.; Fujiwara, T. Enantioselective [2+2]photodimerization reactions of coumarins in solution. Org. Lett. 2005, 7, 1501–1503. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, K. Supramolecular Photodimerization of Coumarins. Molecules 2012, 17, 1408-1418. https://doi.org/10.3390/molecules17021408
Tanaka K. Supramolecular Photodimerization of Coumarins. Molecules. 2012; 17(2):1408-1418. https://doi.org/10.3390/molecules17021408
Chicago/Turabian StyleTanaka, Koichi. 2012. "Supramolecular Photodimerization of Coumarins" Molecules 17, no. 2: 1408-1418. https://doi.org/10.3390/molecules17021408