The Incubation of 13a,17-Dihydroxystemodane with Cephalosporium aphidicola
Abstract
:1. Introduction

2. Results and Discussion
| Position | 3 | 4 | 6a | 6am | 8a | 9a |
|---|---|---|---|---|---|---|
| 1 | 36.2 | 35.8 | 35.7 | 35.9 | 36.0 a | 33.5 |
| 2 | 18.8 | 18.1 | 18.1 | 18.7 | 17.6 | 23.2 |
| 3 | 41.8 | 35.2 | 36.8 | 36.8 | 36.3 b | 74.6 |
| 4 | 33.2 | 37.6 a | 47.2 | 47.7 | 36.5 | 40.8 |
| 5 | 47.2 | 40.4 | 41.6 | 41.9 | 41.1 | 39.5 |
| 6 | 22.2 | 22.0 | 24.6 | 24.6 | 22.1 | 21.6 |
| 7 | 36.6 | 36.2 | 36.7 | 36.7 | 34.6 | 36.1 |
| 8 | 37.3 | 37.2 | 37.7 | 37.7 | 33.6 | 37.2 |
| 9 | 50.7 | 50.8 | 50.6 | 50.6 | 55.0 | 50.9 |
| 10 | 38.5 | 38.3 a | 37.9 | 38.0 | 39.1 | 38.1 |
| 11 | 27.2 | 27.3 | 26.0 | 26.1 | 71.7 | 27.1 |
| 12 | 28.1 | 28.2 | 27.0 | 27.1 | 36.6b | 28.1 |
| 13 | 74.3 | 74.2 | 84.4 | 84.5 | 74.0 | 74.6 |
| 14 | 40.4 | 40.5 | 39.1 | 39.1 | 40.3 | 41.0 |
| 15 | 37.4 | 37.4 | 35.2 | 35.2 | 35.5 a | 37.2 |
| 16 | 29.7 | 29.8 | 29.8 | 29.9 | 28.4 | 29.4 |
| 17 | 68.0 | 68.1 | 64.7 | 64.7 | 69.9 | 69.8 |
| 18 | 34.5 | 72.6 | 182.0 | 179.6 | 73.3 | 65.9 |
| 19 | 22.8 | 18.6 | 17.7 | 17.8 | 18.5 | 13.9 |
| 20 | 18.8 | 19.6 | 19.0 | 19.0 | 20.6 | 19.4 |

3. Experimental
3.1. General Procedures
3.2. Microorganism
3.3. Incubation of 3
4. Conclusions
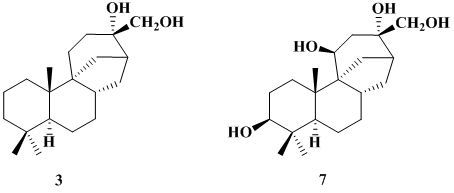
- The hydroxylations produced in the substrate 3 by this fungus occurred at C-3(β), C-11(β) and C-18.
- The hydroxylation at C-18 points to a biosynthetically-directed transformation, since aphidicolin (2) is also hydroxylated at this carbon. This position was also functionalized in the biotransformation of stemodine and stemodinone with C. aphidicola [10,11]. However, the C-3(β) and C-11(β) hydroxylations, also observed in the incubation of 3, seem to indicate a xenobiotic biotransformation. These hydroxylations were also observed in the feeding of 3 with M. plumbeus [13], a fungus used in the latter type.
- The oxidation of C-18 to acid level, as occurs in the formation of 6 from 4, has now been observed for the first time in a biotransformation with C. aphidicola.
- It is probable that the formation of 9 only occurs from 4, and not from 5. Thus the hydroxylation of the C-18 methyl in 5 to form 9 could be inhibited by the presence of the equatorial β-hydroxyl group at C-3 (Scheme 2). In aphidicolin biosynthesis has been noted that an axial α-hydroxyl at C-3 blocks the hydroxylation of C-18 [8], whilst in gibberellin biosynthesis has been observed that an equatorial 3α-OH inhibits hydroxylation of C-19 [16].
Acknowledgements
References and Notes
- Thiericke, R.; Rohr, J. Biological variation of microbial metabolites by precursor-directed biosynthesis. Nat. Prod. Rep. 1993, 10, 265–289. [Google Scholar] [CrossRef]
- Hanson, J.R. The microbiological transformation of diterpenoids. Nat. Prod. Rep. 1992, 9, 139–151. [Google Scholar] [CrossRef]
- Fraga, B.M.; González-Vallejo, V.; Guillermo, R. On the biotransformation of ent-trachylobane to ent-kaur-11-ene diterpenes. J. Nat. Prod. 2011, 74, 1985–1989. [Google Scholar] [CrossRef]
- Fraga, B.M.; González-Vallejo, V.; Guillermo, R.; Díaz, L.N. Biotransformation of 7α-hydroxy- and 7-oxo-ent-atis-16-ene derivatives by the fungus Gibberella fujikuroi. Phytochemistry 2010, 71, 1313–1321. [Google Scholar]
- Hanson, J.R.; Nasir, H.; Parvez, A. The hydroxylation of testosterone and some relatives by Cephalosporium aphidicola. Phytochemistry 1996, 42, 411–415. [Google Scholar]
- Atta-ur-Rahman; Yaqoob, A.; Farooq, A.; Anjun, S.; Fahim, A.; Choudhary, M.I. Fngal transformation of (1R, 2S, 5R)-(−)-menthol by Cephalosporium aphidicola. J. Nat. Prod. 1998, 61, 1340–1342. [Google Scholar] [CrossRef]
- Hanson, J.R.; Jarvis, A.J.; Ratcliffe, A.H. Biotransformation of some aphidicolane derivatives by Cephalosporium aphidicola. Phytochemistry 1992, 31, 3851–3853. [Google Scholar]
- Hanson, J.R.; Jarvis, A.J.; Laboret, F.; Takahashi, J. The incubation of 3α,16β-dihydroxyaphidicolane with Cephalosporium aphidicola. Phytochemistry 1995, 38, 73–75. [Google Scholar]
- Dalziel, W.; Hesp, B.; Stevenson, K.M.; Jarvis, J.A.J. The structure and absolute configuration of the antibiotic aphidicolin: A tetracyclic diterpenoid containing a new ring system. J. Chem. Soc. Perkin Trans. I 1973, 2841–2851. [Google Scholar]
- Badria, F.A.; Hufford, C.D. Microbial transformations of stemodin, a Stemodia diterpene. Phytochemistry 1991, 30, 2265–2268. [Google Scholar]
- Hanson, J.R.; Reese, P.B.; Takahashi, J.A.; Wilson, M.R. Biotransformation of some stemodane diterpenoids by Cephalosporium aphidicola. Phytochemistry 1994, 36, 1391–1393. [Google Scholar]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A.; Gambaro, V. Stemodane diterpenes from Stemodia chilensis. Phytochemistry 1991, 30, 1719–1721. [Google Scholar]
- Fraga, B.M.; Guillermo, R.; Hernández, M.G.; Chamy, M.C.; Garbarino, J.A. Biotransformation of two stemodane diterpenes by Mucor plumbeus. Tetrahedron 2004, 60, 7921–7932. [Google Scholar]
- González, A.G.; Arteaga, J.M.; Bretón, J.L.; Fraga, B.M. Five new labdane diterpene oxides from Eupatorium jhanii. Phytochemistry 1977, 16, 107–110. [Google Scholar]
- González, A.G.; Fraga, B.M.; Hernández, M.G.; Hanson, J.R. The 13C NMR spectra of some ent-18-hydroxy-kaur-16-enes. Phytochemistry 1981, 20, 846–847. [Google Scholar]
- Fraga, B.M.; González, A.G.; Hanson, J.R.; Hernández, M.G. The microbiological transformation of some ent-3β-hydroxykaur-16-enes by Gibberella fujikuroi. Phytochemistry 1981, 20, 57–61. [Google Scholar]
- Sample Availability: Samples of the compounds 3 and 5 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fraga, B.M.; Guillermo, R.; Hernández, M.G.; Chamy, M.C.; Garbarino, J.A. The Incubation of 13a,17-Dihydroxystemodane with Cephalosporium aphidicola. Molecules 2012, 17, 1744-1750. https://doi.org/10.3390/molecules17021744
Fraga BM, Guillermo R, Hernández MG, Chamy MC, Garbarino JA. The Incubation of 13a,17-Dihydroxystemodane with Cephalosporium aphidicola. Molecules. 2012; 17(2):1744-1750. https://doi.org/10.3390/molecules17021744
Chicago/Turabian StyleFraga, Braulio M., Ricardo Guillermo, Melchor G. Hernández, María C. Chamy, and Juan A. Garbarino. 2012. "The Incubation of 13a,17-Dihydroxystemodane with Cephalosporium aphidicola" Molecules 17, no. 2: 1744-1750. https://doi.org/10.3390/molecules17021744