Treatment of Direct Blending Dye Wastewater and Recycling of Dye Sludge
Abstract
:1. Introduction
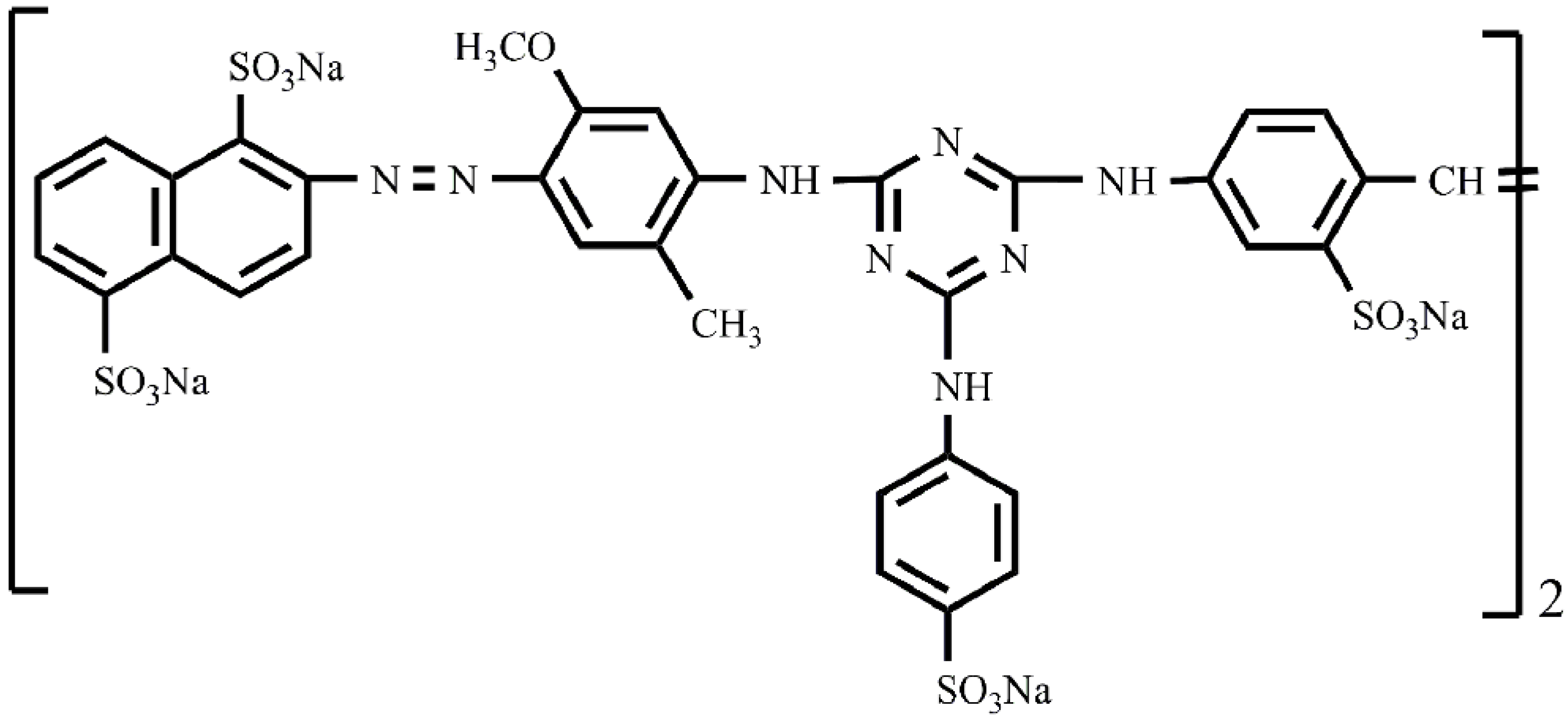
2. Results and Discussion
2.1. Preparation and Characterization of the Dye Conjugate–BaSO4 Hybrid



2.2. Sorption Selectivity
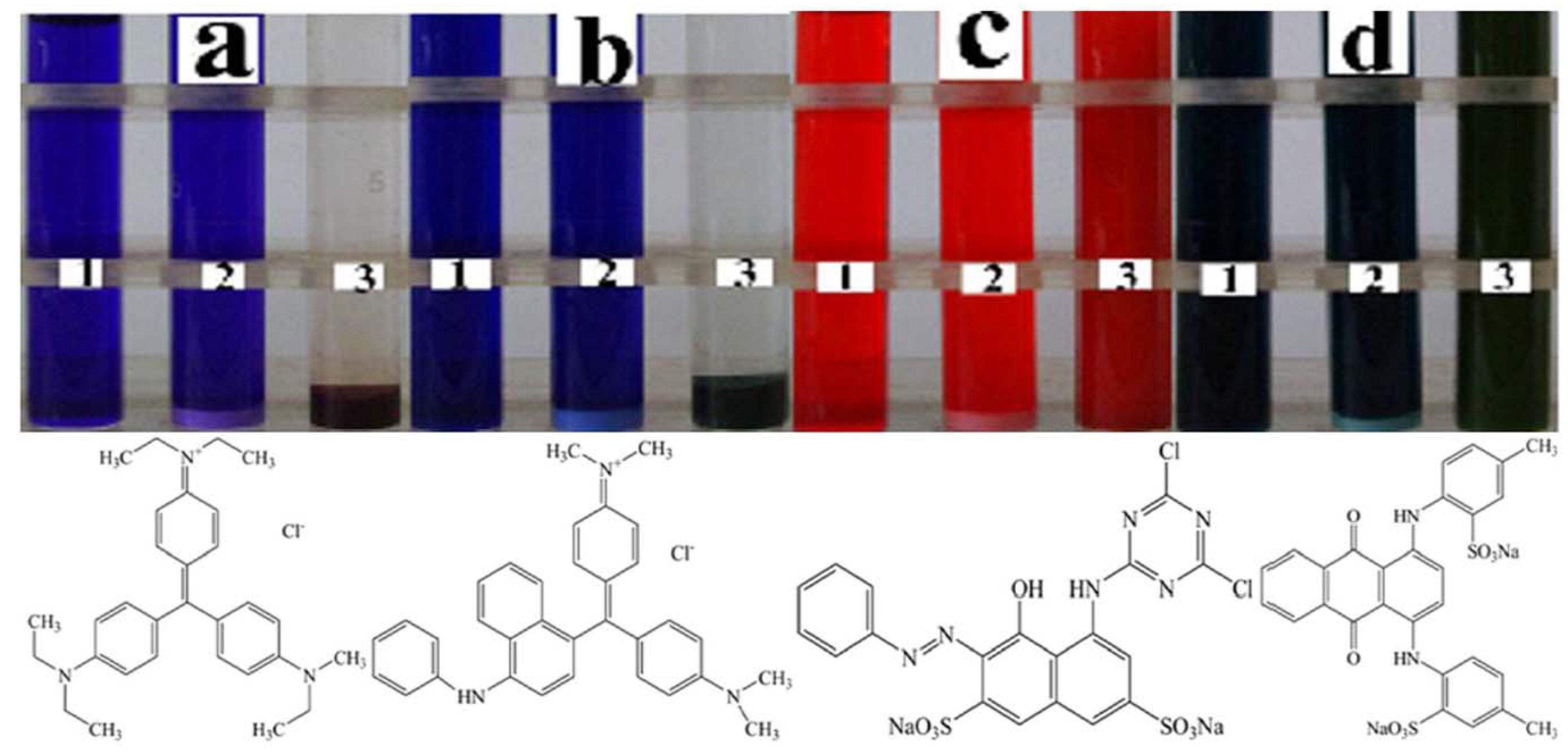
2.3. Sorption Isotherm

2.4. Treatment of Dye Wastewater

2.5. Recycling of the Dye Sludge

3. Experimental
3.1. Apparatus and Materials
3.2. Preparation and Characterization of the Dye Conjugate–BaSO4 Hybrid
3.3 Sorption of Dyes
3.4. Treatment of Dye Wastewater
3.5. Reusing Dye-Contaminated Sludge as Colorants

4. Conclusions
Acknowledgements
References and Notes
- Gong, R.M.; Li, M.; Yang, C.; Sun, Y.Z.; Chen, J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J. Hazard. Mater. 2005, 121, 247–250. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.K.; Vats, P.; Banerjee, U.C. Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit. Rev. Env. Sci. Tec. 2005, 35, 219–238. [Google Scholar] [CrossRef]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Taghavi, S.; van der Lelie, D.; van Aken, B.; Foret, M.; Onderwater, R.C.A.; Wesenberg, D.; et al. Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ. Sci. Technol. 2008, 42, 584–589. [Google Scholar]
- Yang, M.; Yu, J.W.; Li, Z.L.; Guo, Z.H.; Burch, M.; Lin, T.F. Taihu lake not to blame for Wuxi’s woes. Science 2008, 319, 158. [Google Scholar]
- Hadi, M.; Samarghandi, M.R.; McKay, G. Simplified fixed bed design models for the adsorption of acid dyes on novel pine cone derived activated carbon. Water Air Soil Poll. 2011, 218, 197–212. [Google Scholar] [CrossRef]
- Auxilio, A.R.; Andrews, P.C.; Junk, P.C.; Spiccia, V. Adsorption and intercalation of Acid Blue 9 on Mg-Al layered double hydroxides of variable metal composition. Polyhedron 2007, 26, 3479–3490. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Gursoy, B.H.; Schmidt, J.E. Advanced oxidation of acid and reactive dyes: Effect of Fenton treatment on aerobic, anoxic and anaerobic processes. Dyes Pigments 2008, 78, 117–130. [Google Scholar] [CrossRef]
- Sarioglu, M.; Bisgin, T. Removal of Maxilon Yellow GL in a mixed methanogenic anaerobic culture. Dyes Pigments 2007, 75, 544–549. [Google Scholar] [CrossRef]
- Rivera, M.; Pazos, M.; Sanroman, M.A. Improvement of dye electrochemical treatment by combination with ultrasound technique. J. Chem. Technol. Biotechnol. 2009, 84, 1118–1124. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.R.A.; Arami, M. Response surface optimization of acid red 119 dye from simulated wastewater using Al based waterworks sludge and polyaluminium chloride as coagulant. J. Environ. Manag. 2011, 92, 1284–1291. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Tizaoui, C.; Hilal, N. Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desalination 2011, 266, 1–16. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amoros, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrol. 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Phan, N.H.; Rio, S.; Faur, C.; Coq, L.L.; Cloirec, P.L.; Nguyen, T.H. Production of fibrous activated carbons from natural cellulose (jute, coconut) fibers for water treatment applications. Carbon 2006, 44, 2569–2577. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, G.; Kumar, K. Removal of dyes from wastewater using flyash, a low-cost adsorbent. Ind. Eng. Chem. Res. 2002, 41, 3688–3695. [Google Scholar] [CrossRef]
- Meshko, V.; Markovska, L.; Mincheva, M.; Rodrigues, A.E. Adsorption of basic dyes on granular acivated carbon and natural zeolite. Water Res. 2001, 35, 3357–3366. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Removal of cationic and ionic dyes on industrial-municipal sludge based composite adsorbents. Ind. Eng. Chem. Res. 2007, 46, 1786–1793. [Google Scholar] [CrossRef]
- Gurses, A.; Karaca, S.; Dogar, C.; Bayrak, R.; Açıkyıldız, M.; Yalçın, M. Determination of adsorptive properties of clay/water system: Methylene blue sorption. J. Colloid Interf. Sci. 2004, 269, 310–314. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kasai, H.; Nakanishi, H.; Suzuki, T.M. Test strips for heavy-metal ions fabricated from nanosized dye compounds. Angew. Chem. Int. Ed.Engl. 2006, 45, 913–916. [Google Scholar] [CrossRef]
- Xu, C.S.; Kim, H.; Yang, H.; Hayden, C.C. Multiparameter Fluorescence Spectroscopy of Single Quantum Dot−Dye FRET Hybrids. J. Am. Chem. Soc. 2007, 129, 11008–11009. [Google Scholar] [CrossRef]
- Hou, K.; Song, Q.; Nie, D.; Li, F.; Bian, Z.; Liu, L.; Xu, L.; Huang, C. Synthesis of Amphiphilic Dye-Self-Assembled Mesostructured Powder Silica with Enhanced Emission for Directional Random Laser. Chem. Mater. 2008, 20, 3814–3820. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ma, L.M.; Li, T.; Zhang, Y.L.; Gao, H.W. Synthesis of Ag(SCN)/tetrabromo-tetrachlorofluorescein inclusion material and application to synthetic dye. Colloids Surface. A 2009, 333, 126–132. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gao, H.W. Preparation of calcium oxalate—bromopyrogallol red inclusion sorbent and application to treatment of cationic dye and heavy metal wastewaters. Environ. Sci. Pollut. Res. 2009, 16, 339–347. [Google Scholar] [CrossRef]
- Zhao, D.H.; Gao, H.W. Turning calcium carbonate into a cost-effective wastewater-sorbing material by occluding waste dye. Environ. Sci. Pollut. Res. 2010, 17, 95–107. [Google Scholar]
- Zhao, D.H.; Zhang, Y.L.; Wei, Y.P.; Gao, H.W. Facile Eco-friendly Treatment of Dye Wastewater Mixture by In-situ Hybridization with Growing Calcium Carbonate. J. Mater. Chem. 2009, 19, 7239–7244. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Raji, I.O.; Olaseni, S.E.; Onimisi, T.D. In situ hybridization of waste dyes into growing particles of calcium derivatives synthesized from a Gastropod shell (Achatina Achatina). Chem. Eng. J. 2011, 171, 941–950. [Google Scholar] [CrossRef]
- Tan, S.; Tinc, T. Flammability and mechanical properties of Al(OH)3 and BaSO4 filled polypropylene. J. Appl. Polym. Sci. 2010, 118, 3034–3040. [Google Scholar] [CrossRef]
- Wei, Y.P.; Wei, D.Q.; Gao, H.W. Treatment of Dye Wastewater by in-situ Hybridization with Mg-Al Layered Double Hydroxides and Reuse of Dye Sludge. Chem. Eng. J. 2011, 172, 872–878. [Google Scholar] [CrossRef]
- Lin, J.; Gao, H.W. SDBS@BaSO4: An efficient wastewater-sorbing material. J. Mater. Chem. 2009, 19, 3598–3601. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, P.; Shivakumara, C. Synthesis of BaSO4 nanoparticles by precipitation method using sodium hexametaphosphate as a stabilizer. Solid. State. Commun. 2010, 150, 386–388. [Google Scholar] [CrossRef]
- Tal, A. Seeking sustainability: Israel’s evolving water management strategy. Science 2006, 313, 1081–1084. [Google Scholar] [CrossRef]
- Liu, W.Z.; Huang, F.; Liao, Y.Q.; Zhang, J.; Ren, G.Q.; Zhuang, Z.Y.; Zhen, J.S.; Lin, Z.; Wang, C. Treatment of CrVI-containing Mg(OH)2 nanowaste. Angew. Chem. Int. Ed. Engl. 2008, 47, 5619–5622. [Google Scholar]
- Commission Internationale d’Eclairage (CIE), Colorimetry; CIE Pub No.15.2. 2nd ed; Central Bureau of the CIE: Vienna, Austria, 1986; p. 33.
- Faltermeier, A.; Behr, M.; Mussig, D. Esthetic brackets: The influence of filler level on color stability. Am. J. Orthod. Dentofac. 2007, 132, e13–e16. [Google Scholar]
- Doray, P.; Wang, X.; Powers, J.; Burgess, J. Accelerated aging affects color stability of provisional restorative materials. Int. J. Prosthodont. 1996, 6, 183–188. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, X.-H.; Li, M.-L.; Yuan, Y. Treatment of Direct Blending Dye Wastewater and Recycling of Dye Sludge. Molecules 2012, 17, 2784-2795. https://doi.org/10.3390/molecules17032784
Xu X-H, Li M-L, Yuan Y. Treatment of Direct Blending Dye Wastewater and Recycling of Dye Sludge. Molecules. 2012; 17(3):2784-2795. https://doi.org/10.3390/molecules17032784
Chicago/Turabian StyleXu, Xin-Hui, Ming-Li Li, and Yuan Yuan. 2012. "Treatment of Direct Blending Dye Wastewater and Recycling of Dye Sludge" Molecules 17, no. 3: 2784-2795. https://doi.org/10.3390/molecules17032784



