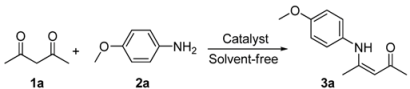
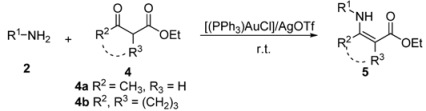
Efficient Synthesis of β-Enaminones and β-Enaminoesters Catalyzed by Gold (I)/Silver (I) under Solvent-Free Conditions
Abstract
:1. Introduction
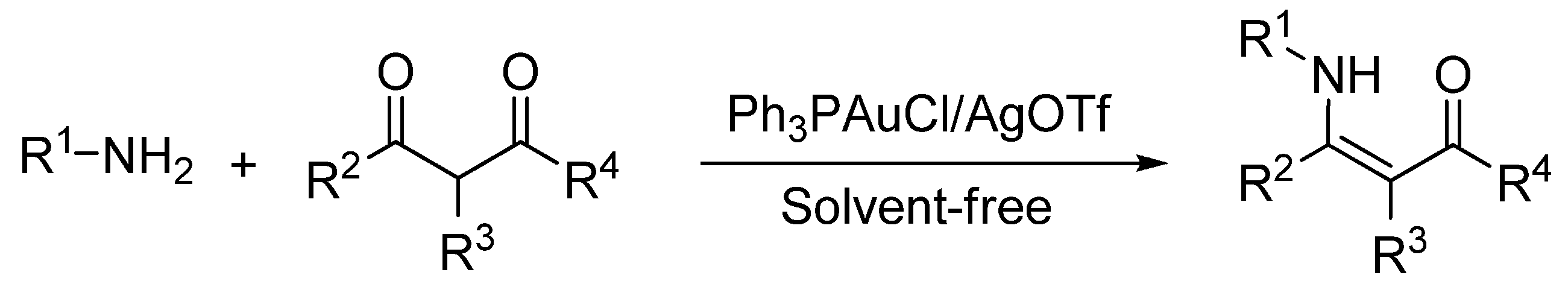
2. Results and Discussion
| Entry | Catalyst | Time (h) | Yield (%) b |
|---|---|---|---|
| 1 | - | 2 | 25 |
| 2 | (PPh3)AuCl | 2 | 33 |
| 3 | AgOTf | 2 | 28 |
| 4 | (PPh3)AuCl + AgOTf | 2 | 98 |
| 5c | (PPh3)AuCl + AgOTf | 6 | 85 |
| Entry | 2 (R1) | Time | 3 | Yield (%) b |
|---|---|---|---|---|
| 1 | 4-CH3OC6H4 | 2 h | 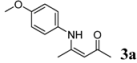 | 98 |
| 2 | C6H5 | 4 h | 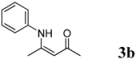 | 85 |
| 3 | 4-CH3C6H4 | 3 h |  | 87 |
| 4 | 4-BrC6H4 | 4 h | 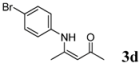 | 90 |
| 5 | 4-ClC6H4 | 5 h |  | 88 |
| 6 | C10H7 | 5 h |  | 96 |
| 7 | Bn | 5 min |  3g 3g | 95 |
| 8 | n-C4H9 | 5 min |  3h 3h | 96 |
| 9 | Allyl | 1.5 h | 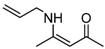 3i 3i | 98 |
| 10 | 2-Hydroxyethyl | 5 min |  3j 3j | 96 |
| Entry | 2 (R1) | 4 | Time | 5 | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 4-CH3OC6H4 | 4a | 3 h | 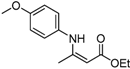 5a 5a | 98 |
| 2 | C6H5 | 4a | 5 h |  5b 5b | 82 |
| 3 | 4-CH3C6H4 | 4a | 4 h | 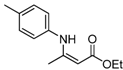 5c 5c | 92 |
| 4 | 4-BrC6H4 | 4a | 5 h | 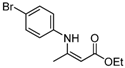 5d 5d | 86 |
| 5 | 4-ClC6H4 | 4a | 5 h | 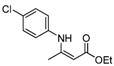 5e 5e | 76 |
| 6 | C10H7 | 4a | 8 h | 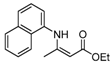 5f 5f | 85 |
| 7 | Bn | 4a | 5 min | 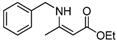 5g 5g | 97 |
| 8 | n-C4H9 | 4a | 5 min |  5h 5h | 95 |
| 9 | Allyl | 4a | 1 h | 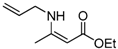 5i 5i | 97 |
| 10 | 2-Hydroxyethyl | 4a | 5 min | 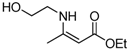 5j 5j | 96 |
| 11 | 4-CH3OC6H4 | 4b | 2 h |  5k 5k | 93 |
| 12 | C6H5 | 4b | 2 h | 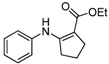 5l 5l | 87 |
| 13 | 4-CH3C6H4 | 4b | 1.5 h | 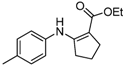 5m 5m | 94 |
| 14 | 4-BrC6H4 | 4b | 2 h | 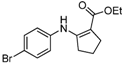 5n 5n | 92 |
| 15 | 4-ClC6H4 | 4b | 2 h | 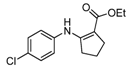 5o 5o | 90 |
| 16 | C10H7 | 4b | 2 h |  5p 5p | 90 |
| 17 | Bn | 4b | 5 min | 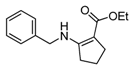 5q 5q | 85 |
3. Experimental
3.1. General
3.2. Preparation of (PPh3)AuCl
3.3. Typical Procedure for the Synthesis of β-Enaminones and β-Enaminoesters
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Reddy, G.J.; Latha, D.; Thirupathaiah, C.; Rao, K.S. A facile synthesis of 2,3-disubstituted-6-arylpyridines from enaminones using montmorillonite K10 as solid acid support. Tetrahedron Lett. 2005, 46, 301–302. [Google Scholar]
- Negri, G.; Kascheres, C.; Kascheres, A.J. Recent developments in the chemistry of enaminones. J. Heterocycl. Chem. 2004, 41, 461–491. [Google Scholar] [CrossRef]
- Abdelkhalik, M.M.; Eltoukhy, A.M.; Agamy, M.; Elnagdi, M.H. A General and Efficient Method for the Preparation of β-Enamino Ketones and Esters Catalyzed by Indium Tribromide. J. Heterocycl. Chem. 2004, 41, 431–434. [Google Scholar] [CrossRef]
- Michael, J.P.; Koning, C.B.; Hosken, G.D.; Stanbury, T.V. Reformatsky reactions with N-arylpyrrolidine-2-thiones: Synthesis of tricyclic analogues of quinolone antibacterial agents. Tetrahedron 2001, 57, 9635–9648. [Google Scholar]
- Azzaro, M.; Geribaldi, S.; Videau, B. Use of Boron Trifluoride Etherate in the Preparation of 2-Amino-1-alkenyl Ketones from β-Diketones and Low-Boiling Amines. Synthesis 1981, 880–881. [Google Scholar]
- Dannhardt, G.; Bauer, A.; Nowe, U. Synthesis and Pharmacological Activity of Enaminones which Inhibit both Bovine Cyclooxygenase and 5-Lipoxygenase. J. Prakt. Chem. 1998, 340, 256–263. [Google Scholar] [CrossRef]
- Boger, D.L.; Ishizaki, T.; Wysocki, J.R.J.; Munk, S.A.; Kitos, P.A.; Suntornwat, O. Total synthesis and evaluation of (±)-N-(tert-butoxycarbonyl)-CBI, (±)-CBI-CDPI1, and (±)-CBI-CDPI2: CC-1065 functional agents incorporating the equivalent 1,2,9,9a-tetrahydrocyclopropa[1,2-c]benz[1,2-e]indol-4-one (CBI) left-hand subunit. J. Am. Chem. Soc. 1989, 111, 6461–6463. [Google Scholar]
- Haycock-Lewandowski, S.J.; Wilder, A.; Ahman, J. Development of a Bulk Enabling Route to Maraviroc (UK-427,857), a CCR-5 Receptor Antagonist. J. Org. Process Res. Dev. 2008, 12, 1094–1103. [Google Scholar] [CrossRef]
- Cimarelli, C.; Palmieri, G. Stereoselective Reduction of Enantiopure β-Enamino Esters by Hydride: A Convenient Synthesis of Both Enantiopure β-Amino Esters. J. Org. Chem. 1996, 61, 5557–5563. [Google Scholar] [CrossRef]
- Potin, D.; Dumas, F.; Angelo, J.D. New chiral auxiliaries: Their use in the asymmetric hydrogenation of β-acetamidocrotonates. J. Am. Chem. Soc. 1990, 112, 3483–3486. [Google Scholar] [CrossRef]
- Cimarelli, C.; Palmieri, G.; Volpini, E. An Improved Synthesis of Enantiopure β-Amino Acids. Synth. Commun. 2001, 31, 2943–2953. [Google Scholar] [CrossRef]
- Beholz, L.G.; Benovsky, R.; Ward, D.L.; Bata, N.S.; Stille, J.R. Formation of Dihydropyridone- and Pyridone-Based Peptide Analogs through Aza-Annulation of β-Enamino Ester and Amide Substrates with α-Amido Acrylate Derivatives. J. Org. Chem. 1997, 62, 1033–1042. [Google Scholar]
- Nakamura, I.; Yamamoto, Y. Transition-Metal-Catalyzed Reactions in Heterocyclic Synthesis. Chem. Rev. 2004, 104, 2127–2198. [Google Scholar]
- Chaaban, I.; Greenhill, J.V.; Akhtar, P. Enaminones in the mannich reaction. Part 2. Further investigations of internal mannich reactions. J. Chem. Soc. Perkin Trans. I 1979, 1593–1596. [Google Scholar]
- Figueiredo, L.J.O.; Kascheres, C. Quinone Diazides and Enaminones as a Source of New Azo Compounds with Potential Nonlinear Optical Properties. J. Org. Chem. 1997, 62, 1164–1167. [Google Scholar] [CrossRef]
- Aceña, J.L.; Arjona, O.; Mañas, R.; Plumet, J. Unexpected One-Pot Epoxy Sulfone-Enaminone Transformation. Synthesis of 5α-Carba-β-mannopyranosylamine. J. Org. Chem. 2000, 65, 2580–2582. [Google Scholar]
- Li, G.; Watson, K.; Buckheit, R.W.; Zhang, Y. Total Synthesis of Anibamine, a Novel Natural Product as a Chemokine Receptor CCR5 Antagonist. Org. Lett. 2007, 9, 2043–2046. [Google Scholar] [CrossRef]
- Calle, M.; Calvo, L.A.; Ortega, A.G.; Gonzalez-Nogal, A.M. Silylated β-enaminones as precursors in the regioselective synthesis of silyl pyrazoles. Tetrahedron 2006, 62, 611–618. [Google Scholar]
- Ferraz, H.M.C.; Pereira, F.L.C.; Leite, F.S.; Nuns, M.R.S.; Payret-Arrua, M.E. Synthesis of N-substituted pyrrole and tetrahydroindole derivatives from alkenyl β-dicarbonyl compounds. Tetrahedron 1999, 55, 10915–10924. [Google Scholar]
- Khosropour, A.R.; Khodaei, M. A mild, efficient and environmentally friendly method for the regio- and chemoselective synthesis of enaminones using Bi(TFA)3 as a reusable catalyst in aqueous media. Tetrahedron Lett. 2004, 45, 1725–1728. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, T.S.; Li, J.J. Synthesis of enaminones and enamino esters catalysed by ZrOCl2·8H2O. Catal. Commun. 2007, 8, 1615–1620. [Google Scholar] [CrossRef]
- Li, G.C. Simple and Efficient Synthesis of 3-Aminopropenones and 3-Aminopropenoates Catalyzed by Copper (II) Nitrate Trihydrate under Solvent-Free Conditions. Monatsh. Chem. 2008, 139, 789–792. [Google Scholar] [CrossRef]
- Xu, S.L.; Li, C.P.; Li, J.H. Solid-State Synthesis of β-Enamino Ketones from Solid 1,3-Dicarbonyl Compounds and Ammonium Salts or Amines. Synlett 2009, 5, 818–822. [Google Scholar]
- Chen, X.; She, J.; Shang, Z.C.; Wu, J.; Wu, H.F.; Zhang, P.Z. Synthesis of Pyrazoles, Diazepines, Enaminones, and Enamino Esters Using 12-Tungstophosphoric Acid as a Reusable Catalyst in Water. Synthesis 2008, 21, 3478–3486. [Google Scholar]
- Rafiee, E.; Joshaghani, M.; Eavania, S.; Rashidzadeh, S. A revision for the synthesis of β-enaminones in solvent free conditions: efficacy of different supported heteropoly acids as active and reusable catalysts. Green Chem. 2008, 10, 982–989. [Google Scholar] [CrossRef]
- Sridharan, V.; Avendaño, C.; Menéndez, J.C. General, Mild and Efficient Synthesis of β-Enaminones Catalyzed by Ceric Ammonium Nitrate. Synlett 2007, 6, 881–884. [Google Scholar]
- Arcadi, A.; Bianchi, G.; Giuseppe, S.D.; Marinelli, F. Gold catalysis in the reactions of 1,3-dicarbonyls with nucleophiles. Green Chem. 2003, 5, 64–67. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yin, L.; Wang, Y.M. Tandem β-Enamino Ester Formation and Cyclization with o-Alkynyl Anilines Catalyzed by InBr3: Efficient Synthesis of β-(N-Indolyl)-α,β-unsaturated Esters. Adv. Synth. Catal. 2006, 348, 184–190. [Google Scholar] [CrossRef]
- Krishna, P.R.; Sekhar, E.R. p-Toluenesulfonylmethyl Isocyanide (TosMIC) and Indium Manifold Strategy to Access β-Keto-(E)-enamino Esters from 1,3-Dicarbonyl Compounds. Adv. Synth. Catal. 2008, 350, 2871–2876. [Google Scholar] [CrossRef]
- Kidwai, M.; Bhardwaj, S.; Mishra, N.K.; Bansal, V.; Kumar, A.; Mozumdar, S. A novel method for the synthesis of β-enaminones using Cu-nanoparticles as catalyst. Catal. Commun. 2009, 10, 1514–1517. [Google Scholar] [CrossRef]
- Harrad, M.A.; Outtouch, R.; Ali, M.A.; Firdoussi, L.E.; Karim, A.; Roucoux, A. Ca(CF3COO)2: An efficient Lewis acid catalyst for chemo- and regio-selective enamination of β-dicarbonyl compounds. Catal. Commun. 2010, 11, 442–446. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ma, Z.C.; Mo, L.P. Enamination of 1,3-dicarbonyl compounds catalyzed by tin tetrachloride. Indian J. Chem. 2007, 46B, 535–539. [Google Scholar]
- Stefani, H.A.; Costa, I. M.; Silva, D.D. An Easy Synthesis of Enaminones in Water as Solvent. Synthesis 2000, 1526–1528. [Google Scholar]
- Khodaei, M.M.; Khosropour, A.R.; Kookhazadeh, M. A novel enamination of β-dicarbonyl compounds catalyzed by Bi(TFA)3 immobilized on molten TBAB. Can. J. Chem. 2005, 83, 209–212. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef]
- Nieto-Oberhuber, C.; Muñoz, M.P.; Buñuel, E.; Nevado, C.; Cárdenas, D.J.; Echavarren, A.M. Cationic Gold(I) Complexes: Highly Alkynophylic Catalysts for the Exo- and Endo-Cyclization of Enynes. Angew. Chem. Int. Ed. Engl. 2004, 43, 2402–2406. [Google Scholar]
- Brandys, M.C.; Jennings, M.C.; Puddephatt, R.J. Luminescent gold(I) macrocycles with diphosphine and 4,4’-bipyridyl ligands. J. Chem. Soc. Dalton Trans. 2000, 4601–4606. [Google Scholar]
- Ito, H.; Saito, T.; Miyahara, T.; Zhong, C.; Sawamura, M. Gold(I) Hydride Intermediate in Catalysis: Dehydrogenative Alcohol Silylation Catalyzed by Gold(I) Complex. Organometallics 2009, 28, 4829–4840. [Google Scholar] [CrossRef]
- Metzger, J.O. Solvent-Free Organic Syntheses. Angew. Chem. Int. Ed.Engl. 1998, 37, 2975–2978. [Google Scholar] [CrossRef]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar]
- Cave, G.W.V.; Raston, C.L.; Scotta, J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 37, 2159–2169. [Google Scholar]
- Mézailles, N.; Ricard, L.; Gagosz, F. Phosphine Gold(I) Bis-(trifluoromethanesulfonyl)imidate Complexes as New Highly Efficient and Air-Stable Catalysts for the Cycloisomerization of Enynes. Org. Lett. 2005, 7, 4133–4136. [Google Scholar] [CrossRef]
- Sanguramath, R.A.; Hooper, T.N.; Butts, C.P.; Green, M.; McGrady, J.E.; Russell, C.A. The Interaction of Gold(I) Cations with 1,3-Dienes. Angew. Chem. Int. Ed. Engl. 2011, 50, 7592–7595. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, M.; Abdukader, A.; Fu, Y.; Zhu, C. Efficient Synthesis of β-Enaminones and β-Enaminoesters Catalyzed by Gold (I)/Silver (I) under Solvent-Free Conditions. Molecules 2012, 17, 2812-2822. https://doi.org/10.3390/molecules17032812
Zhang M, Abdukader A, Fu Y, Zhu C. Efficient Synthesis of β-Enaminones and β-Enaminoesters Catalyzed by Gold (I)/Silver (I) under Solvent-Free Conditions. Molecules. 2012; 17(3):2812-2822. https://doi.org/10.3390/molecules17032812
Chicago/Turabian StyleZhang, Ming, Ablimit Abdukader, Yong Fu, and Chengjian Zhu. 2012. "Efficient Synthesis of β-Enaminones and β-Enaminoesters Catalyzed by Gold (I)/Silver (I) under Solvent-Free Conditions" Molecules 17, no. 3: 2812-2822. https://doi.org/10.3390/molecules17032812