Synthesis and Preliminary Investigations of the siRNA Delivery Potential of Novel, Single-Chain Rigid Cationic Carotenoid Lipids
Abstract
:1. Introduction


2. Results
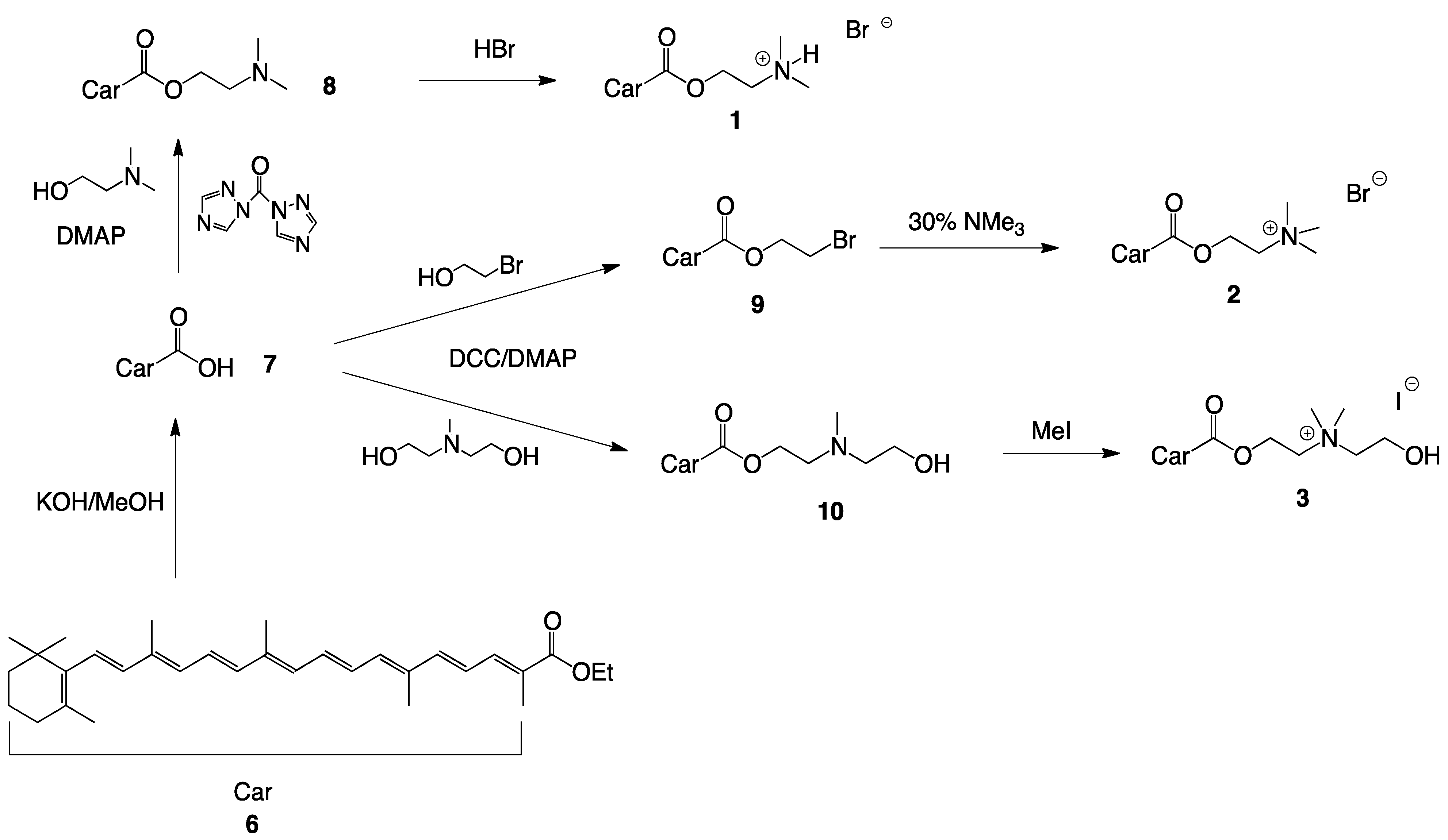
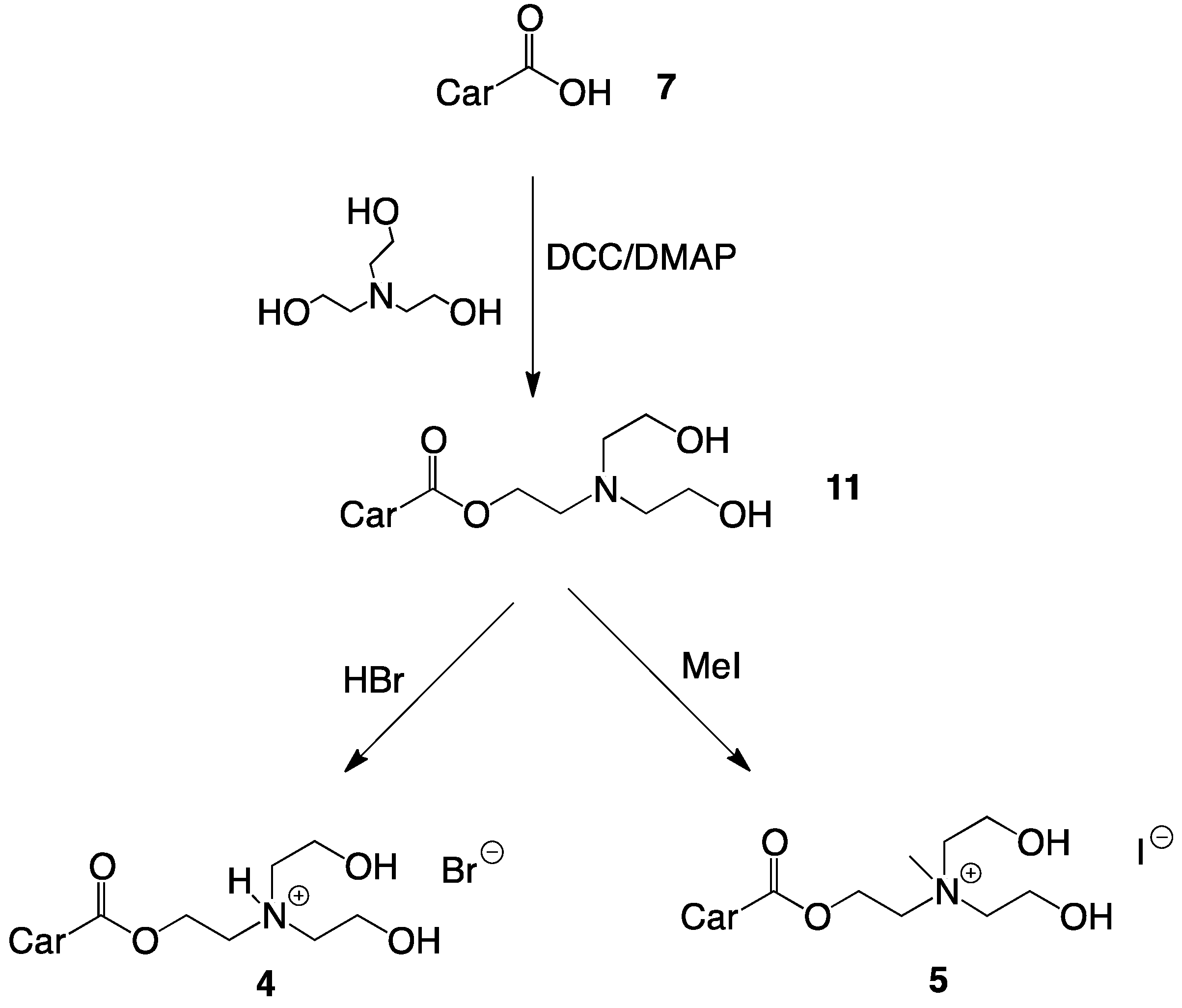
2.1. Synthesis of Single-Chain Cationic Carotenoid Lipids, 1–5
2.2. Lipid/siRNA Lipoplex Formulation and Particle Sizing

2.3. Lipid/siRNA Transfection

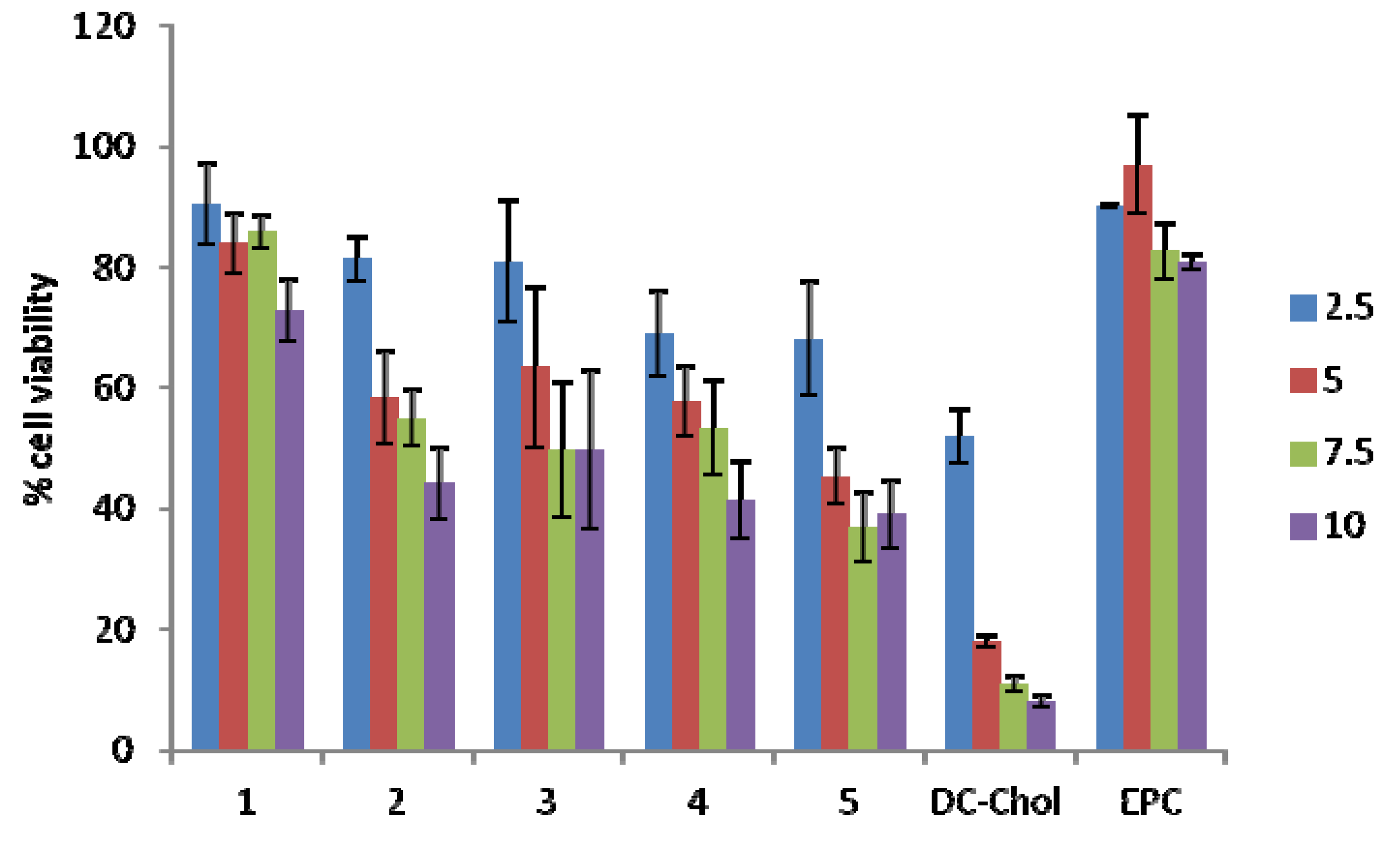
2.4. Cellular Toxicity
3. Discussion
4. Experimental
4.1. Materials
4.2. SiRNA Sequences
4.3. General Methods
4.4. Synthesis
4.4.1. Synthesis of β-apo-8´-Carotenoic Acid (7)
4.4.2. Synthesis of 2-(N,N-Dimethylamino)ethyl-β-apo-8′-carotenoate (8)
4.4.3. Synthesis of 2-(N,N-Dimethylamino)ethyl-β-apo-8'-carotenoate hydrobromide (1)
4.4.4. Synthesis of 2-Bromoethyl-β-apo-carotenoate (9)
4.4.5. Synthesis of 2-(N,N,N-Trimethyl) ethyl-β-apo-8'-carotenoate bromide (2)
4.4.6. Synthesis of 2-(N-(2-Hydroxyethyl), N-Methylamino)ethyl-β-apo-8'-carotenoate (10)
4.4.7. Synthesis of 2-(N-(2-Hydroxyethyl), N,N-Dimethylammonium)ethyl-β-apo-8'-carotenoate iodide (3)
4.4.8. Synthesis of 2-(N,N-Di(2-hydroxyethyl)amino)ethyl-β-apo-8'-carotenoate (11)
4.4.9. Synthesis of 2-(N,N-Di(2-hydroxyethyl)amino)ethyl-β-apo-8'-carotenoate hydrobromide (4)
4.4.10. Synthesis of 2-(N,N-Di(2-hydroxyethyl), N-methylammonium)ethyl-β-apo-8'-carotenoate Iodide (5)
4.5. Particle Formulation Methods
4.6. Bioassay Methods
5. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Veldhoen, S.; Laufer, S.D.; Restle, T. Recent developments in peptide-based nucleic acid delivery. Int. J. Mol. Sci. 2008, 9, 1276–1320. [Google Scholar] [CrossRef]
- Hattori, Y. Development of non-viral vector for cancer gene therapy. Yakugaku Zasshi 2010, 130, 917–923. [Google Scholar] [CrossRef]
- Lentacker, I.; Vandenbroucke, R.E.; Lucas, B.; Demeester, J.; de Smedt, S.C.; Sanders, N.N. New strategies for nucleic acid delivery to conquer cellular and nuclear membranes. J. Control. Release 2008, 132, 279–288. [Google Scholar] [CrossRef]
- de Ilarduya, C.T.; Sun, Y.; Duzguneş, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S. Cell transfection by DNA-lipid complexes—Lipoplexes. Biochemistry 2009, 74, 1293–1304. [Google Scholar]
- He, C.X.; Tabata, Y.; Gao, J.Q. Non-viral gene delivery carrier and its three-dimensional transfection system. Int. J. Pharm. 2010, 386, 232–242. [Google Scholar] [CrossRef]
- Maitani, Y.; Igarashi, S.; Sato, M.; Hattori, Y. Cationic liposome (DC-Chol/DOPE = 1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int. J. Pharm. 2007, 342, 33–39. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishikawa, M.; Takakura, Y. Nonviral vector-mediated RNA interference: Its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv. Drug Deliv. Rev. 2009, 61, 760–766. [Google Scholar] [CrossRef]
- Nguyen, T.; Menocal, E.M.; Harborth, J.; Fruehauf, J.H. RNAi therapeutics: An update on delivery. Curr. Opin. Mol. Ther. 2008, 10, 158–167. [Google Scholar]
- Karagiannis, T.C.; El-Osta, A. RNA interference and potential therapeutic applications of short interfering RNAs. Cancer Gene Ther. 2005, 12, 787–795. [Google Scholar] [CrossRef]
- Kim, H.K.; Davaa, E.; Myung, C.S.; Park, J.S. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int. J. Pharm. 2010, 392, 141–147. [Google Scholar] [CrossRef]
- Hirko, A.; Tang, F.; Hughes, J.A. Cationic lipid vectors for plasmid DNA delivery. Curr. Med. Chem. 2003, 10, 1185–1193. [Google Scholar] [CrossRef]
- Hoekstra, D.; Rejman, J.; Wasungu, L.; Shi, F.; Zuhorn, I. Gene delivery by cationic lipids: In and out of an endosome. Biochem. Soc. Trans. 2007, 35, 68–71. [Google Scholar] [CrossRef]
- Ross, P.C.; Hui, S.W. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999, 6, 651–659. [Google Scholar] [CrossRef]
- Kennedy, M.T.; Pozharski, E.V.; Rakhmanova, V.A.; MacDonald, R.C. Factors governing the assembly of cationic phospholipid-DNA complexes. Biophys. J. 2000, 78, 1620–1633. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef]
- Escriou, V.; Ciolina, C.; Lacroix, F.; Byk, G.; Scherman, D.; Wils, P. Cationic lipid-mediated gene transfer: effect of serum on cellular uptake and intracellular fate of lipopolyamine/DNA complexes. Biochim. Biophys. Acta 1998, 1368, 276–288. [Google Scholar] [CrossRef]
- Niculescu-Duvaz, D.; Heyes, J.; Springer, C.J. Structure-activity relationship in cationic lipid mediated gene transfection. Curr. Med. Chem. 2003, 10, 1233–1261. [Google Scholar] [CrossRef]
- Bouxsein, N.F.; McAllister, C.S.; Ewert, K.K.; Samuel, C.E.; Safinya, C.R. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry 2007, 46, 4785–4792. [Google Scholar] [CrossRef]
- Bottega, R.; Epand, R.M. Inhibition of protein kinase C by cationic amphiphiles. Biochemistry 1992, 31, 9025–9030. [Google Scholar] [CrossRef]
- Arakawa, K.; Eguchi, T.; Kakinuma, K. Tightly packed membranes composed of 36-membered macrocyclic diether phospholipid found in archaea growing under deep-sea hydrothermal vents. Chem. Lett. 1998, 901–902. [Google Scholar]
- Lemoine, N.R. Understanding Gene Therapy; BIOS scientific publishers: Oxford, UK, 1999. [Google Scholar]
- Larsen, E.; Abendroth, J.; Partali, V.; Schulz, B.; Sliwka, H.-R.; Quartey, E.G.K. Combination of vitamin E with a carotenoid: α-Tocopherol and trolox linked to β-apo-8′-carotenoic acid. Chem. Eur. J. 1998, 4, 113–117. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Reimer, D.L.; Zhang, G.; Lee, P.H.; Bally, M.B. Self-assembling DNA-lipid particles for gene transfer. Pharm. Res. 1997, 14, 190–196. [Google Scholar] [CrossRef]
- Gaucheron, J.; Wong, T.; Wong, K.F.; Maurer, N.; Cullis, P.R. Synthesis and properties of novel tetraalkyl cationic lipids. Bioconjug. Chem. 2002, 13, 671–675. [Google Scholar] [CrossRef]
- Eastman, S.J.; Siegel, C.; Tousignant, J.; Smith, A.E.; Cheng, S.H.; Scheule, R.K. Biophysical characterization of cationic lipid: DNA complexes. Biochim. Biophys. Acta 1997, 1325, 41–62. [Google Scholar] [CrossRef]
- Dass, C.R.; Walker, T.L.; Burton, M.A. Liposomes containing cationic dimethyl dioctadecyl ammonium bromide: Formulation, quality control, and lipofection efficiency. Drug Deliv. 2002, 9, 11–18. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Liu, F.; Huang, L. Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv. Drug Del. Rev. 2005, 57, 689–698. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, F.; Li, Z.; Li, S.; Huang, L. Sequential injection of cationic liposome and plasmid DNA effectively transfects the lung with minimal inflammatory toxicity. Mol. Ther. 2001, 3, 673–682. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar]
- Banerjee, R.; Das, P.K.; Srilakshmi, G.V.; Chaudhuri, A.; Rao, N.M. Novel series of non-glycerol-based cationic transfection lipids for use in liposomal gene delivery. J. Med. Chem. 1999, 42, 4292–4299. [Google Scholar] [CrossRef]
- Byk, G.; Wetzer, B.; Frederic, M.; Dubertret, C.; Pitard, B.; Jaslin, G.; Scherman, D. Reduction-sensitive lipopolyamines as a novel nonviral gene delivery system for modulated release of DNA with improved transgene expression. J. Med. Chem. 2000, 43, 4377–4387. [Google Scholar] [CrossRef]
- Sample Availability: Not avaliable.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pungente, M.D.; Jubeli, E.; Øpstad, C.L.; Al-Kawaz, M.; Barakat, N.; Ibrahim, T.; Khalique, N.A.; Raju, L.; Jones, R.; Leopold, P.L.; et al. Synthesis and Preliminary Investigations of the siRNA Delivery Potential of Novel, Single-Chain Rigid Cationic Carotenoid Lipids. Molecules 2012, 17, 3484-3500. https://doi.org/10.3390/molecules17033484
Pungente MD, Jubeli E, Øpstad CL, Al-Kawaz M, Barakat N, Ibrahim T, Khalique NA, Raju L, Jones R, Leopold PL, et al. Synthesis and Preliminary Investigations of the siRNA Delivery Potential of Novel, Single-Chain Rigid Cationic Carotenoid Lipids. Molecules. 2012; 17(3):3484-3500. https://doi.org/10.3390/molecules17033484
Chicago/Turabian StylePungente, Michael D., Emile Jubeli, Christer L. Øpstad, Mais Al-Kawaz, Nour Barakat, Tarek Ibrahim, Nada Abdul Khalique, Liji Raju, Rachel Jones, Philip L. Leopold, and et al. 2012. "Synthesis and Preliminary Investigations of the siRNA Delivery Potential of Novel, Single-Chain Rigid Cationic Carotenoid Lipids" Molecules 17, no. 3: 3484-3500. https://doi.org/10.3390/molecules17033484



