Determination of Rutin in Cigarette Tobacco, Filters, Mainstream Smoke and Burned Ash of Different Branded Cigarettes by High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussions
2.1. Optimization of the Solvent to Solid Ratio
2.2. Optimization of the Extraction Time

2.3. Effect of Ultrasound Power
2.4. Optimization of HPLC Method

2.5. Preparation of Calibration Curve
| Compound | Regression equation | R2 | Linearity range
(mg/mL) | LOD
(μg/mL) | LOQ
(μg/mL) |
|---|---|---|---|---|---|
| rutin | y = 17438 x – 24155 | 0.9998 | 0.03–0.93 | 10.88 | 32.63 |
2.6. Precision
2.7. Reproducibility Test
2.8. Recovery Test
| Component | Original amount (μg) | Amount spiked (μg) | Amount found (μg) | peak height
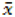 (μv) (μv) | Standard deviation σ (min) | Recovery (%) | R.S.D. (%) |
|---|---|---|---|---|---|---|---|
| rutin | 4.15 ± 0.11 | 3.26 ± 0.13 | 7.39 ± 0.23 | 36985 ± 866 | 0.17 ± 0.01 | 99.19 ± 0.35 | 1.58 ± 0.21 |
| 4.15 ± 0.11 | 4.18 ± 0.20 | 8.37 ± 0.19 | 42153 ± 539 | 0.25 ± 0.02 | 100.84 ± 0.47 | 2.64 ± 0.91 | |
| 4.15 ± 0.11 | 4.93 ± 0.16 | 9.01 ± 0.50 | 43379 ± 933 | 0.24 ± 0.02 | 98.47 ± 0.34 | 2.29 ± 0.59 |
| Brands | Contents (μg/per cigarette) | |||
|---|---|---|---|---|
| Tobacco | Filter | Mainstream smoke | Burned ash | |
| brand 1 | 49.42 ± 0.90 b | 0.19 ± 0.01 d | 0.14 ± 0.00 b | ND |
| brand 2 | 20.76 ± 0.64 d | 0.23 ± 0.02 c | 0.13 ± 0.01 bc | ND |
| brand 3 | 20.84 ± 0.75 d | 0.14 ±0.00 f | 0.06 ± 0.01 e | ND |
| brand 4 | 15.55 ± 1.25 e | 0.22 ± 0.01 c | 0.12 ± 0.01 c | ND |
| brand 5 | 27.57 ± 1.03 c | 0.26 ± 0.01 b | 0.08 ± 0.01 d | ND |
| brand 6 | 17.38 ± 1.34 e | 0.32 ± 0.01 a | 0.16 ± 0.02 a | ND |
| brand 7 | 10.20 ± 0.30 f | 0.15 ± 0.01 e | 0.12 ± 0.01 c | ND |
| brand 8 | 29.21 ± 1.77 c | 0.16 ± 0.01 e | 0.08 ± 0.01 d | ND |
| brand 9 | 28.79 ± 1.16 c | 0.14 ± 0.01 ef | 0.08 ± 0.01 d | ND |
| brand 10 | 63.98 ± 2.43 a | 0.10 ± 0.01 g | 0.07 ± 0.01 de | ND |
2.9. Application
3. Experimental
3.1. Reagents and Materials
3.2. Instrument and Chromatography Conditions
3.3. Sample Preparation

3.4. Preparation of Standard Solution
4. Conclusions
Acknowledgements
References and Notes
- Wu, D.; Landsberger, S.; Larson, S.M. Determination of the elemental distribution in cigarette componentsand smoke by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 1997, 217, 77–82. [Google Scholar]
- Carmines, E.L. Evaluation of the potential effects of ingredients added to cigarettes. Part 1: Cigarette design, testing approach, and review of results. Food Chem. Toxicol. 2002, 40, 77–91. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Davis, D.; Nielsen, M.T. Tobacco: Production, Chemistry and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 1999. [Google Scholar]
- Wang, H.Y.; Zhao, M.M.; Yang, B.; Jiang, Y.M.; Rao, G.H. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar]
- Weisburger, J.H.; Chung, F.L. Mechanisms of chronic disease causation by nutritional factors and tobacco products and their prevention by tea polyphenols. Food Chem. Toxicol. 2002, 40, 1145–1154. [Google Scholar]
- Qiu, J.S. Statistics aided optimization for high-performance liquid chromatographic analysis of organic acids in tobacco. J. Chromatogr. A 1999, 859, 153–158. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, W.S.; Shao, X.G. Determination of seven polyphenols in water by high performance liquidchromatography combined with preconcentration. Talanta 2008, 77, 679–683. [Google Scholar]
- Xiang, G.; Yang, H.Y.; Yang, L.; Zhang, X.; Cao, Q.U.; Miao, M.M. Multivariate statistical analysis of tobacco of different origin, grade and variety according to polyphenols and organic acids. Microchem. J. 2010, 95, 198–206. [Google Scholar]
- Chen, S.; Gong, J.; Liu, F.; Mohammed, U. Naturally occurring polyphenolicantioxidants modulate IgE-mediated mast cell activation. Immunology 2000, 100, 471–480. [Google Scholar]
- Ihme, N.; Kiesewetter, H.; Jung, F.; Hoffmann, K.H.; Birk, A.; Muller, A.; Grutzner, K.I. Leg oedema protection from a buckwheat herb tea in patientswith chronic venous insufficiency: A single-centre, randomised, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Pharmacol. 1996, 50, 443–447. [Google Scholar]
- Deschner, E.E.; Ruperto, J.; Wong, G.; Newmark, H.L. Quercetin and rutinas inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis 1991, 12, 1193–1196. [Google Scholar]
- Panasiak, W.; Wleklik, M.; Oraczewska, A.; Luczak, M. Influence of flavonoidson combined experimental infections withEMCvirus and Staphylococcus aureusin mice. Acta Microbiol. Pol. 1989, 38, 185–188. [Google Scholar]
- Zhao, S.N.; Kwok, K.C.; Liang, H.H. Investigation on ultrasound assisted extraction ofsaikosaponins from Radix Bupleuri. Sep. Purif. Technol. 2007, 55, 307–312. [Google Scholar] [CrossRef]
- Dong, J.E.; Liu, Y.B.; Liang, Z.S.; Wang, W.L. Investigation on ultrasound-assisted extraction of salvianolic acid Bfrom Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar]
- Xiao, W.H.; Han, L.J.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 616–620. [Google Scholar]
- Zhao, L.C.; Liang, J.; Li, W.; Cheng, K.M.; Xia, X.H.; Deng, X.; Yang, G.L. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules 2011, 16, 5928–5937. [Google Scholar]
- Wu, D.; Landsberger, S.; Larson, S.M. Determination of the elemental distribution in cigarette components and smoke by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 1997, 217, 77–82. [Google Scholar]
- Djordjevic, M.V.; Stellman, S.D.; Zang, E. Doses of dicotine and lung carcinogens delivered to cigarette smokers. J. Natl. Cancer Inst. 2000, 92, 106–111. [Google Scholar]
- Thomas, E.M.; Anthony, P.B.; Naren, K.M.; Chan, W.G. Phenolic compound formation from the low temperature pyrolysis of tobacco. J. Anal. Appl. Pyrol. 2009, 84, 170–178. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, Y.; Li, W.; Wang, J.; Bi, J.; Su, S. Determination of Rutin in Cigarette Tobacco, Filters, Mainstream Smoke and Burned Ash of Different Branded Cigarettes by High Performance Liquid Chromatography. Molecules 2012, 17, 3751-3760. https://doi.org/10.3390/molecules17043751
Sun Y, Li W, Wang J, Bi J, Su S. Determination of Rutin in Cigarette Tobacco, Filters, Mainstream Smoke and Burned Ash of Different Branded Cigarettes by High Performance Liquid Chromatography. Molecules. 2012; 17(4):3751-3760. https://doi.org/10.3390/molecules17043751
Chicago/Turabian StyleSun, Yinshi, Wei Li, Jianhua Wang, Jianjie Bi, and Shudong Su. 2012. "Determination of Rutin in Cigarette Tobacco, Filters, Mainstream Smoke and Burned Ash of Different Branded Cigarettes by High Performance Liquid Chromatography" Molecules 17, no. 4: 3751-3760. https://doi.org/10.3390/molecules17043751




