Phytotoxic Effects and a Phytotoxin from the Invasive Plant Xanthium italicum Moretti
Abstract
:1. Introduction

2. Results and Discussion
2.1. Phytotoxicity Assays of Different Plant Parts of X. italicum
2.2. Phytotoxic Effects of Organic Extracts of X. italicum Fruit
| Amaranth | Lettuce | Wheat | Ryegrass | |||||
|---|---|---|---|---|---|---|---|---|
| Root length | Shoot length | Root length | Shoot length | Root length | Shoot length | Root length | Shoot length | |
| control | 2.21 ± 0.26 a | 1.28 ± 0.08 a | 4.53 ± 0.36 a | 2.03 ± 0.06 a | 12.13 ± 0.56 a | 4.76 ± 0.05 a | 3.55 ± 0.10 a | 2.66 ± 0.06 a |
| stem | 1.00 ± 0.13 c | 1.29 ± 0.08 a | 1.83 ± 0.09 d | 2.25 ± 0.05 a | 8.63 ± 0.48 bc | 4.18 ± 0.03 b | 1.94 ± 0.22 c | 1.78 ± 0.20 bc |
| leaf | 0 d | 0 c | 0 e | 0 b | 0 d | 0 c | 0 d | 0 d |
| fruit | 0 d | 0 c | 0 e | 0 b | 0 d | 0 c | 0 d | 0 d |
| root | 1.00 ± 0.10 c | 0.89 ± 0.06 b | 2.41 ± 0.15 c | 1.98 ± 0.10 a | 7.74 ± 0.32 c | 4.43 ± 0.10 ab | 2.48 ± 0.21 b | 1.40 ± 0.15 c |
| litter | 1.63 ± 0.06 b | 1.34 ± 0.05 a | 3.78 ± 0.15 b | 2.08 ± 0.08 a | 9.92 ± 0.27 b | 4.22 ± 0.22 b | 2.60 ± 0.19 b | 1.98 ± 0.15 b |
| Amaranth | Lettuce | Wheat | Ryegrass | |||||
|---|---|---|---|---|---|---|---|---|
| Root length | Shoot length | Root length | Shoot length | Root length | Shoot length | Root length | Shoot length | |
| control | 2.21 ± 0.26 a | 1.28 ± 0.08 b | 4.53 ± 0.36 a | 2.03 ± 0.06 b | 12.13 ± 0.56 a | 4.76 ± 0.05 b | 3.55 ± 0.10 a | 2.66 ± 0.06 a |
| Petroleum ether | 1.15 ± 0.15 c | 0.71 ± 0.09 c | 4.85 ± 0.14 a | 2.23 ± 0.04 b | 8.14 ± 0.59 b | 4.37 ± 0.11 b | 3.14 ± 0.24 ab | 2.45 ± 0.17 a |
| Chloroform | 0.10 ± 0.00 e | 0.10 ± 0.10 d | 0.71 ± 0.08 c | 0.23 ± 0.05 d | 0.92 ± 0.13 c | 1.48 ± 0.12 d | 0.03 ± 0.03 d | 0.77 ± 0.15 bc |
| Ethyl acetate | 1.68 ± 0.18 b | 1.15 ± 0.14 b | 4.68 ± 0.18 a | 2.13 ± 0.04 b | 10.13 ± 0.29 a | 4.26 ± 0.15 b | 3.24 ± 0.10 a | 2.35 ± 0.09 a |
| n-butanol | 0.58 ± 0.11 d | 0.69 ± 0.09 c | 2.90 ± 0.08 b | 1.60 ± 0.04 c | 7.73 ± 0.15 b | 3.45 ± 0.07 c | 1.04 ± 0.06 c | 1.04 ± 0.10 b |
| Water phase | 1.58 ± 0.26 b | 2.39 ± 0.26 a | 3.25 ± 0.13 b | 3.35 ± 0.08 a | 11.34 ± 0.90 a | 5.52 ± 0.18 a | 2.88 ± 0.35 b | 2.35 ± 0.17 a |
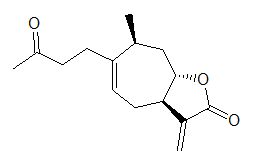
2.3. Identification of an Allelochemical from X. italicum Fruit

2.4. Phytotoxic Effects of Purified Xanthinosin


3. Experimental
3.1. General
3.2. Plant Material
3.3. Phytotoxic Effects of Ethanol Extracts of Different Plant Parts
3.4. Phytotoxic Effects of Organic Extracts of Fruits
3.5. Extraction and Isolation
3.6. Phytotoxic Effects of Purified Xanthinosin
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
References and Notes
- Liu, Q.R.; Yu, M.; Zhou, Y.L. A preliminary study on the invasive plants in Beijing. J. Beijing Normal Univ. (Nat. Sci.) 2002, 38, 399–404. [Google Scholar]
- Li, N.; Zhu, L.N.; Zhai, Q.; Zhu, M.W.; Chen, X.H.; Qu, B. A new alien invasive plant—Xanthium italicum Moretti in Liaoning Province. Plant Quar. 2010, 24, 49–52. [Google Scholar]
- Wang, R.; Wan, F.H. Prediction of the potential survival area of Xanthium Italicumin China. Acta Pratacult. Sin. 2010, 19, 222–230. [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China, The Directory of Imported Plant Quarantine Pests of the People’s Republic of China; The Ministry of Agriculture Bulletin No. 862 of the People’s Republic of China: Beijing, China, 2007.
- Zhao, J. Physical Geography of China; Higher Education Press: Beijing, China, 1995. [Google Scholar]
- Takakura, K.; Fujii, S. Reproductive interference and salinity tolerance differentiate habitat use between two alien cockleburs: Xanthium occidentale and X. italicum (Compositae). Plant Ecol. 2010, 206, 309–319. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Antonio, C.M.D.; Loop, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Kitayama, K.; Mueller-Dombois, D. Biological invasion on an oceanic island mountain: Do alien plant species have wider ecological ranges than native species? J. Veg. Sci. 1995, 6, 667–674. [Google Scholar] [CrossRef]
- Goodall, D.W.; Perry, R.A. Arid Land Ecosystems: Volume 2, Structure, Functioning and Management; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Holzmueller, E.; Jose, S. Invasion success of cogongrass, an alien C4 perennial grass, in the southeastern United States: Exploration of the ecological basis. Biol. Invasions 2011, 13, 435–442. [Google Scholar] [CrossRef]
- Blair, A.C.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. A lack of evidence for an ecological role of the putative allelochemical (+/−)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006, 32, 2327–2331. [Google Scholar]
- Duke, S.O.; Blair, A.C.; Dayan, F.E.; Johnson, R.D.; Meepagala, K.M.; Cook, D.; Bajsa, J. Is (−)-catechin a novel weapon of spotted knapweed (Centaurea stoebe)? J. Chem. Ecol. 2009, 35, 141–153. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–433. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Thelen, G.C.; Diaconu, A.; Callaway, R.M. Root exudate is allelopathic in invaded community but not in native community: Field evidence for the novel weapons hypothesis. J. Ecol. 2009, 97, 641–645. [Google Scholar]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar]
- Yan, J.; Bi, H.H.; Liu, Y.Z.; Zhang, M.; Zhou, Z.Y.; Tan, J.W. Phenolic Compounds from Merremia umbellata subsp. orientalis and their allelopathic effects on Arabidopsis seed germination. Molecules 2010, 15, 8241–8250. [Google Scholar] [CrossRef]
- Shao, H.; Peng, S.L.; Wei, X.Y.; Zhang, D.Q.; Zhang, C. Potential allelochemicals from an invasive weed Mikania micrantha H.B.K. J. Chem. Ecol. 2005, 31, 1657–1668. [Google Scholar] [CrossRef]
- Ens, E.J.; Bremner, J.B.; French, K.; Korth, J. Identification of volatile compounds released by roots of an invasive plant, bitou bush (Chrysanthemoides monilifera spp. rotundata), and their inhibition of native seedling growth. Biol. Invasions 2009, 11, 275–287. [Google Scholar] [CrossRef]
- Xie, L.J.; Zeng, R.S.; Bi, H.H.; Song, Y.Y.; Wang, R.L.; Su, Y.J.; Chen, M.; Chen, S.; Liu, Y.H. Allelochemical mediated invasion of exotic plants in China. Allelopathy J. 2010, 25, 31–50. [Google Scholar]
- Bohlmann, F.; Jakupovic, J.; Schuster, A. Further eudesmanolides and xanthanolides from Telekia speciosa. Phytochemistry 1981, 20, 1891–1893. [Google Scholar] [CrossRef]
- Bohlmann, F.; Mahanta, P.K.; Jakupovic, J.; Rastogi, R.; Natu, A.A. New sesquiterpene lactones from Inula species. Phytochemistry 1978, 17, 1165–1172. [Google Scholar]
- Marco, J.A.; Sanz-cervera, J.F.; Corral, J.; Carda, M.; Jakupovic, J. Xanthanolides from Xanthium: Absolute configuration of xanthanol, isoxanthanol and their C-4 epimers. Phytochemistry 1993, 34, 1569–1576. [Google Scholar] [CrossRef]
- Irving, R.; Huang, Y.; Hickie, R.A.; Sutherland, R.G.; Barl, B. Xanthatin and xanthinosin from the burs of Xanthium strumarium L. as potential anticancer agents. Can. J. Physiol. Pharmacol 2007, 85, 1160–1172. [Google Scholar] [CrossRef]
- Lavault, M.; Landreau, A.; Larcher, G.; Bouchara, J.P.; Pagniez, F.; Pape, P.L.; Richomme, P. Antileishmanial and antifungal activities of xanthanolides isolated from Xanthium macrocarpum. Fitoterapia 2005, 76, 363–366. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lim, H.J.; Lee, H.J.; Kim, H.; Jeon, R.; Ryu, J. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by xanthanolides isolated from Xanthium strumarium. Bioorg. Med. Chem. Lett. 2008, 18, 2179–2182. [Google Scholar]
- Vasas, A.; Hohmann, J. Xanthane sesquiterpenoids: Structure, synthesis and biological activity. Nat. Prod. Rep. 2011, 28, 824–842. [Google Scholar]
- Sato, Y.; Oketani, H.; Yamada, T.; Singyouchi, K.; Ohtsubo, T.; Kihara, M.; Higuti, T. A xanthanolide with potent antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Pharm. Pharmacol. 1997, 49, 1042–1044. [Google Scholar]
- Favier, L.S.; María, A.O.M.; Wendel, G.H.; Borkowski, E.J.; Giordano, O.S.; Pelzer, L.; Tonn, C.E. Anti-ulcerogenic activity of xanthanolide sesquiterpenes from Xanthium cavanillesii in rats. J. Ethnopharmacol. 2005, 100, 260–267. [Google Scholar] [CrossRef]
- Kovacs, A.; Vasas, A.; Forgo, P.; Rethy, B.; Zupko, I.; Hohmann, J. Xanthanolides with antitumour activity from Xanthium italicum. Z. Naturforsch. C 2009, 64, 343–349. [Google Scholar]
- Tsankova, E.T.; Trendafilova, A.B. Xanthanolides of Xanthium italicum Moretti and their biological activity. Z. Naturforsch. C 1994, 49, 154–155. [Google Scholar]
- Geissman, T.A.; Deuel, P.; Bonde, E.K.; Addicott, F.A. Xanthinin: A plant growth-regulating compound from Xanthium pennsylvanicum. J. Am. Chem. Soc. 1954, 76, 685–687. [Google Scholar] [CrossRef]
- Yokotani-Tomita, K.; Kato1, J.; Yamada1, K.; Kosemura, S.; Yamamura, S.; Bruinsma, J.; Hasegawa1, K. 8-Epixanthatin, a light-induced growth inhibitor, mediates the phototropic curvature in sunflower (Helianthus annuus) hypocotyls. Physiol. Plant. 1999, 106, 326–330. [Google Scholar] [CrossRef]
- Leao, N.P.; Pereira, A.R.; Liu, W.; Ng, J.; Pevzner, P.A.; Dorrestein, P.C.; Konig, G.M.; Vasconcelos, V.M.; Gerwick, W.H. Synergistic allelochemicals from a freshwater cyanobacterium. Proc. Natl. Acad. Sci. USA 2007, 107, 11183–11188. [Google Scholar]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar]
- Grayson, B.T.; Williams, K.S.; Freehauf, P.A.; Pease, R.R.; Ziesel, W.T.; Sereno, R.L.; Reinsfelder, R.E. The physical and chemical properties of the herbicide cinmethylin (SD 95481). Pestic. Sci. 1987, 143–153. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shao, H.; Huang, X.; Wei, X.; Zhang, C. Phytotoxic Effects and a Phytotoxin from the Invasive Plant Xanthium italicum Moretti. Molecules 2012, 17, 4037-4046. https://doi.org/10.3390/molecules17044037
Shao H, Huang X, Wei X, Zhang C. Phytotoxic Effects and a Phytotoxin from the Invasive Plant Xanthium italicum Moretti. Molecules. 2012; 17(4):4037-4046. https://doi.org/10.3390/molecules17044037
Chicago/Turabian StyleShao, Hua, Xiaoli Huang, Xiaoyi Wei, and Chi Zhang. 2012. "Phytotoxic Effects and a Phytotoxin from the Invasive Plant Xanthium italicum Moretti" Molecules 17, no. 4: 4037-4046. https://doi.org/10.3390/molecules17044037