Regioselectivity in the Ring Opening of Epoxides for the Synthesis of Aminocyclitols from D-(-)-Quinic Acid
Abstract
:1. Introduction


2. Results and Discussion



| Compound | H1 ( J)/C1 | H2 ( J)/C2 | H3 ( J)/C3 | H4 ( J)/C4 | H5 ( J)/C5 | H6 ( J)/C6 |
|---|---|---|---|---|---|---|
| 3 | 4.33–4.30 (m) 74.7 | 3.92 (dd, 7.0, 5.3) 81.4 | 4.04–4.01 (m) 69.3 | 3.21 (dd, 10.0, 2.7) 86.8 | 4.07–4.04 (m) 71.9 | 2.25 (dt, 15.7, 3.6) 1.93 (ddd, 15.7, 7.1, 3.7) 32.1 |
| 4 | 4.30 (dt, 6.5, 2.3) 74.1 | 3.89 (dd, 7.6, 5.2) 81.0 | 3.38 (dd, 10.4, 7.6) 76.1 | 3.04 (t, 10.2) 71.5 | 3.62 (td, 11.2, 4.9) 68.6 | 2.34 (ddd, 14.9, 4.9, 2.2) 1.75 (ddd, 14.9, 11.5, 4.1) 34.1 |
| 8 | 3.88 (ddd, 11.6, 10.1, 4.7) 65.3 | 3.95 (dd, 10.0, 3.4) 71.1 | 3.85 (t, 3.5) 64.5 | 3.79 (t, 3.0) 72.5 | 3.74 (ddd, 11.6, 5.2, 3.0) 67.6 | 1.79–1.74 (m) 1.71 (t, 11.6) 32.8 |
| 9 | 3.51–3.40 (m) 66.1 | 3.78 (tm, 9.5, 0.8) 75.0 | 3.32 (td, 9.4, 0.8) 72.3 | 3.13 (td, 9.5, 1.7) 73.0 | 3.45–3.42 (m) 69.7 | 2.01 (dt, 12.1, 4.6) 1.51 (ddd, 13.3, 12.3, 1.4) 36.3 |
| Compound | H1 ( J)/C1 | H2 ( J)/C2 | H3 ( J)/C3 | H4 ( J)/C4 | H5 ( J)/C5 | H6 ( J)/C6 |
|---|---|---|---|---|---|---|
| 5 | 4.03 (dd, 6.3, 3.1) 70.0 | 3.45 (dd, 9.4, 2.8) 73.8 | 3.90 (t, 9.8) 67.2 | 3.12 (d, 10.3, 3.2) 56.7 | 4.09 (dd, 6.5, 3.2) 66.9 | 2.08 (dt, 15.6, 3.5) 1.70 (dt, 15.6, 2.9) 32.6 |
| 6 | 3.96–3.94 (m) 68.3 | 3.41–3.34 (m)a 73.9 | 3.41–3.34 (m)a 71.7 | 2.53 (t, 9.8) 59.4 | 3.59 (ddd, 14.5, 10.0, 4.6) 67.6 | 1.98 (dt, 14.0, 4.2) 1.47 (td, 14.0, 2.5) 36.3 |
| 10 | 3.91 (dt, 9.1, 3.5) 67.0 | 3.75–3.65 (m)a 68.3 | 3.75–3.65 (m)a 67.2 | 3.12 (t, 4.0) 52.6 | 3.75–3.65 (m)a 72.1 | 1.85 (td, 13.1, 4.1) 1.75–1.62 (m) 32.9 |
| 11 | 3.39 (ddd, 11.9, 9.4, 4.6) 68.6 | 3.15 (t, 9.3) 77.0 | 2.99 (t, 9.6) 74.0 | 2.49 (d, 9.8) 58.8 | 3.30 (td, 11.4, 4.4) 68.7 | 2.06 (dt, 12.2, 4.5) 1.35 (dd, 11.9, 11.9) 68.6 |
3. Experimental
3.1. General Methods
3.2. General Procedure of Ring Opening
3.3. General Procedures of Hydrogenation and Deprotection
3.4. Synthesis of the Key Intermediates and the Target Molecules
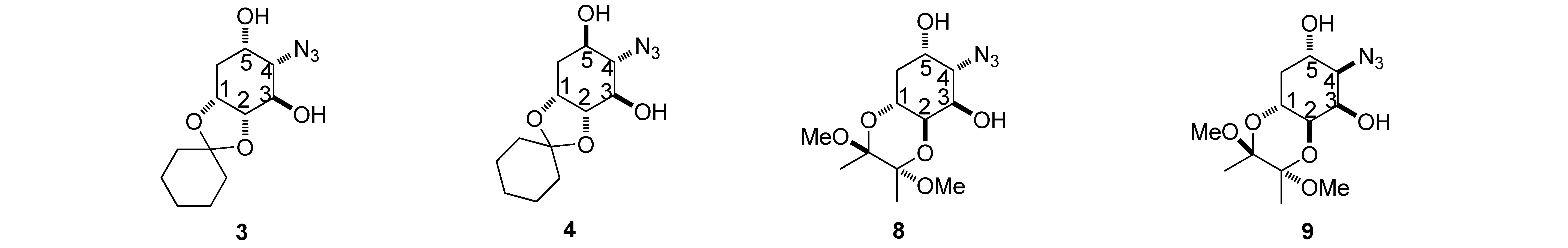
3.4.1. (1R,2R,3R,4S,5S)-4-Azido-1,2-O-cyclohexylidene-cyclohexane-1,2,3,5-tetraol (3)
3.4.2. (1R,2R,3R,4S,5R)-4-Azido-1,2-O-cyclohexylidene-cyclohexane-1,2,3,5-tetraol (4)
3.4.3. (1R,2S,3R,4S,5S)-4-Azido-1,2-[(2S,3S)-2,3-dimethoxybutan-2,3-dioxy]-cyclohexane-1,2,3,5-tetraol (8)
3.4.4. (1R,2S,3R,4R,5S)-4-Azido-1,2-[(2S,3S)-2,3-dimethoxybutan-2,3-dioxy]-cyclohexane-1,2,3,5-tetraol (9)
3.4.5. (1R,2R,3R,4S,5S)-4-Aminocyclohexane-1,2,3,5-tetraol (5)
3.4.6. (1R,2R,3R,4S,5R)-4-Aminocyclohexane-1,2,3,5-tetraol (6)
3.4.7. (1R,2S,3R,4S,5S)-4-Aminocyclohexane-1,2,3,5-tetraol (10)
3.4.8. (1R,2S,3R,4R,5S)-4-Aminocyclohexane-1,2,3,5-tetraol (11)
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Arjona, O.; Gomez, A.M.; Lopez, J.C.; Plumet, J. Synthesis and Conformational and Biological Aspects of Carbasugars. Chem. Rev. 2007, 107, 1919–2036. [Google Scholar]
- Nomenclature of Cyclitols. Available online: http://www.chem.qmul.ac.uk/iupac/cyclitol (accessed on 15 April 2012).
- Duchek, J.; Adams, D.R.; Hudlicky, T. Chemoenzymatic synthesis of inositol, conduritols, and cyclitol analogues. Chem. Rev. 2011, 111, 4223–4258. [Google Scholar] [CrossRef]
- Delgado, A. Recent advances in the chemistry of aminocyclitols. Eur. J. Org. Chem. 2008, 2008, 3893–3906, and references cited therein.. [Google Scholar] [CrossRef]
- Mahmud, T. The C7N aminocyclitol family of natural products. Nat. Prod. Rep. 2003, 20, 137–166. [Google Scholar]
- Dam, J.H.; Madsen, R. Convergent synthesis of pancratistatin from piperonal and xylose. Eur. J. Org. Chem. 2009, 2009, 4666–4673, references cited therein.. [Google Scholar] [CrossRef]
- Sullivan, B.; Carrera, I.; Drouin, M.; Hudlicky, T. Symmetry-based design for the chemoenzymatic synthesis of oseltamivir (Tamiflu) from ethyl benzoate. Angew.Chem. Int. Ed. 2009, 48, 4229–4231. [Google Scholar]
- Gupta, P.; Pal, A.P.J.; Reddy, Y.S.; Vankar, Y.D. Synthesis of aminocyclitols and trihydroxylated indolizidinone from a D-mannitol-derived common building block. Eur. J. Org. Chem. 2011, 2011, 1166–1175. [Google Scholar]
- Ahmad, S.; Thomas, L.H.; Sutherland, A. Stereoselective synthesis of polyhydroxylated aminocyclohexanes. Org. Biomol. Chem. 2011, 9, 2801–2808. [Google Scholar]
- Harrak, Y.; Barra, C.M.; Delgado, A.; Castaña, A.R.; Llebaria, A. Galacto-configured aminocyclitol phytoceramides are potent in vivo invariant natural killer T cell stimulators. J. Am. Chem. Soc. 2011, 133, 12079–12084. [Google Scholar]
- Díaz, L.; Casas, J.; Bujons, J.; Llebaria, A.; Delgado, A. New glucocerebrosidase inhibitors by exploration of chemical diversity of N-substituted aminocyclitols using click chemistry and in situ screening. J. Med. Chem. 2011, 54, 2069–2079. [Google Scholar]
- Díaz, L.; Bujons, J.; Casas, J.; Llebaria, A.; Delgado, A. Click chemistry approach to new N-substituted aminocyclitols as potential pharmacological chaperones for gaucher disease. J. Med. Chem. 2010, 53, 5248–5255. [Google Scholar]
- Kurbanoğlu, N.I.; Celik, M.; Kilic, H.; Alp, C.; Sahin, E.; Balci, M. Stereospecific synthesis of a DL-gala-aminoquercitol derivative. Tetrahedron 2010, 66, 3485–3489. [Google Scholar]
- Pandey, G.; Tiwari, K.N.; Puranik, V.G. Use of enantiomerically Pure 7-azabicyclo[2.2.1]heptan-2-ol as a chiral template for the synthesis of aminocyclitols. Org. Lett. 2008, 10, 3611–3614. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, Y.-H.; Yao, Z.-J. Parallel synthesis of individual shikimic acid-like molecules using a mixture-operation strategy and ring-closing enyne metathesis. Tetrahedron Lett. 2007, 48, 3511–3515. [Google Scholar]
- Alegret, C.; Benet-Buchholz, J.; Riera, A. Stereodivergent syntheses of conduramines and aminocyclitols. Org. Lett. 2006, 8, 3069–3072. [Google Scholar]
- Donohoe, T.; Johnson, P.D.; Pye, R.J.; Keenan, M. Concise and enantioselective synthesis of the aminocyclitol core of hygromycin A. Org. Lett. 2005, 7, 1275–1277. [Google Scholar]
- Shih, T.-L.; Li, H.-Y.; Ke, M.-S.; Kuo, W.-S. Synthesis of a new family of aminocyclitols from D-(−)-quinic acid. Synth.Commun. 2008, 38, 4139–4149. [Google Scholar]
- Shih, T.-L.; Lin, Y.-L. Epoxidation of Protected (1,4,5)-cyclohex-2-ene-triols and their acid hydrolysis to synthesize quercitols from D-(−)-quinic acid. Synth.Commun. 2005, 35, 1809–1817. [Google Scholar]
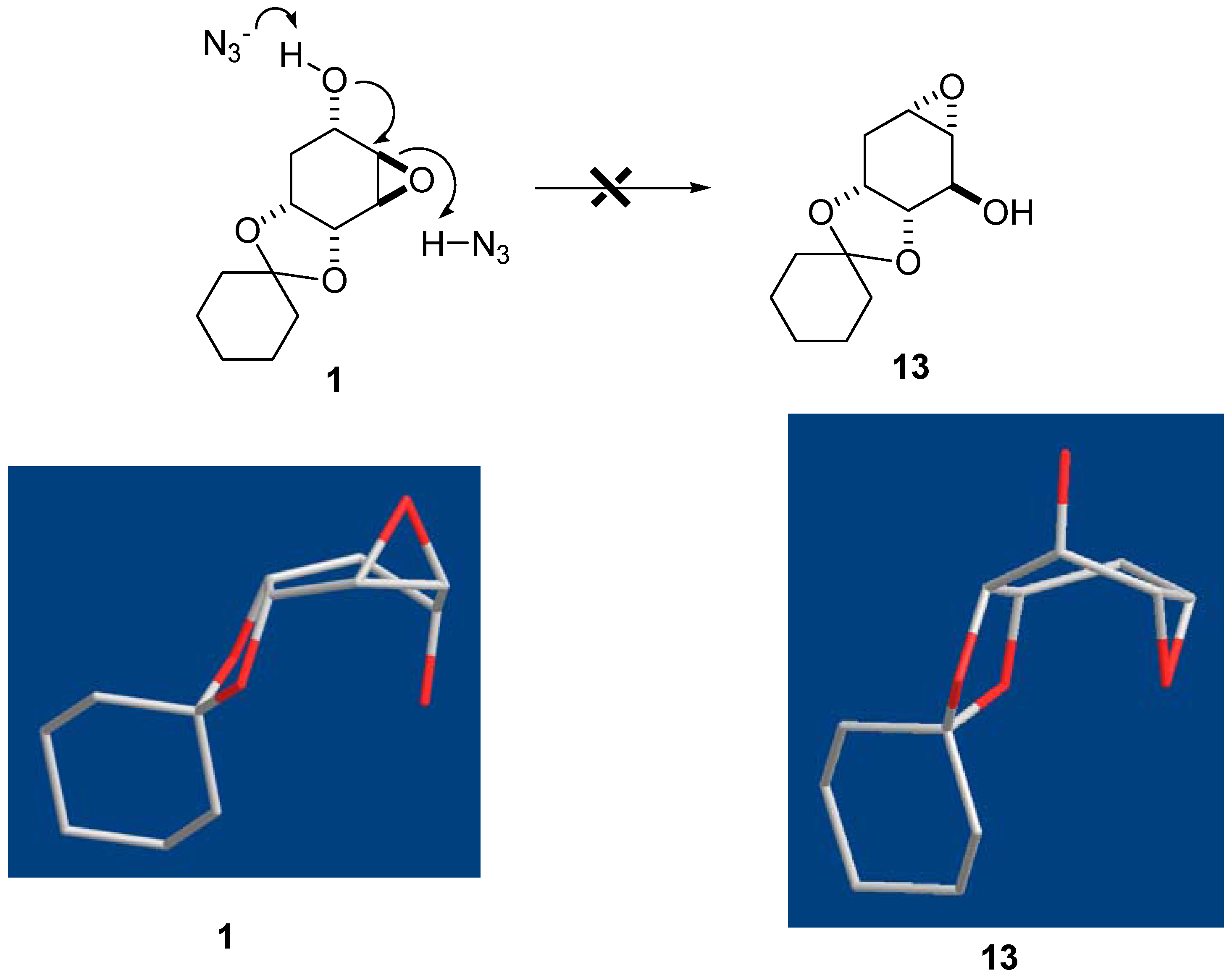
- The crude mixture in reaction of molecule 1 by NaN3 was measured by NMR spectra. An unidentified molecule with approximated 1.6% amount of 3 was detected. We suspected it was the other regioisomer but we failed to isolate it from column chromatography. On the other hand, we did not detect other isomers in reaction of molecule 2. The moderate yields obtained of 3 and 4 from 1 and 2, respectively, were due to their slight decomposition during reactions.
- Payne, G.B. Epoxide migrations with a,β-epoxy alcohols. J. Org. Chem. 1962, 27, 3819–3822. [Google Scholar] [CrossRef]
- Behrens, C.H.; Sharpless, K.B. New transformations of 2,3-epoxy alcohols and related derivatives Easy routes to homochiral substances. Aldrichim. Acta 1983, 16, 67–79. [Google Scholar]
- The NMR data and optical rotation values of compounds 5 and 6 were reported to be dissolved in CDCl3 and MeOH, respectively (see reference 8). But, in general, these compounds and other related analogues are more soluble in D2O or CD3OD than in CDCl3. The NMR data and HRMS are satisfied for molecules 5 and 6.
- Sample Availability: Samples of the compounds 1−11 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shih, T.-L.; Yang, S.-Y. Regioselectivity in the Ring Opening of Epoxides for the Synthesis of Aminocyclitols from D-(-)-Quinic Acid. Molecules 2012, 17, 4498-4507. https://doi.org/10.3390/molecules17044498
Shih T-L, Yang S-Y. Regioselectivity in the Ring Opening of Epoxides for the Synthesis of Aminocyclitols from D-(-)-Quinic Acid. Molecules. 2012; 17(4):4498-4507. https://doi.org/10.3390/molecules17044498
Chicago/Turabian StyleShih, Tzenge-Lien, and Shu-Yu Yang. 2012. "Regioselectivity in the Ring Opening of Epoxides for the Synthesis of Aminocyclitols from D-(-)-Quinic Acid" Molecules 17, no. 4: 4498-4507. https://doi.org/10.3390/molecules17044498