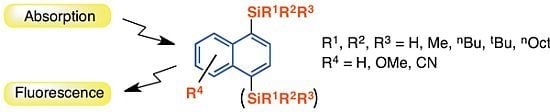
Absorption and Fluorescence Spectroscopic Properties of 1- and 1,4-Silyl-Substituted Naphthalene Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Silyl-Substituted Naphthalene Derivatives
2.2. Effects of Hydrogen Atom and Alkyl Group Containing Substituents on the Absorption and Fluorescence Properties of Substituted Naphthalenes
2.3. Absorption and Fluorescence Properties of Mono- and Di-silyl Substituted Naphthalene Derivatives
2.4. Effects of Electron-Donating and Withdrawing Groups on Absorption and Fluorescence Properties
2.5. Effects of Silylethynyl Group(s) on Absorption and Fluorescence Properties
2.6. Fluorescence Quenching of 9,10-Dicyanoanthracene by Silylnaphthalenes
2.7. HOMO-LUMO Energy Calculations, Fluorescence Lifetimes, and Fluorescence Quantum Yields
3. Experimental
3.1. General
3.2. Preparation of Silyl-Substituted Naphthalene Derivatives
4. Conclusions
Acknowledgements
References and Notes
- Steinmetz, M.G. Organosilane Photochemistry. Chem. Rev. 1995, 95, 1527–1588. [Google Scholar] [CrossRef]
- Miller, R.D.; Michl, J. Polysilane High Polymers. Chem. Rev. 1989, 89, 1359–1410. [Google Scholar] [CrossRef]
- West, R.; Fink, M.J.; Michl, J. Tetramesityldisilene, a Stable Compound Containing a Silicon-Silicon Double Bond. Science 1981, 214, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Collins, S.; Masamune, S. Photo-Induced Fragmentation of Tri-t-butyl-trimethylsilylcyclotrisilanes and E,Z-Interconversion of Di-t-butyldimethylsilyldisilenes. Tetrahedron Lett. 1984, 25, 2131–2134. [Google Scholar] [CrossRef]
- Michalczyk, M.J.; West, R.; Michl, J. Kinetics of Thermal Cis-Trans Isomerizations in Disilenes. Organometallics 1985, 4, 826–829. [Google Scholar] [CrossRef]
- Dewan, J.C.; Murakami, S.; Snow, J.T.; Collins, S.; Masamune, S. Crystal Structures of Two Isomeric Cyclotrisilanes. J. Chem. Soc. Chem. Commun. 1985, 892–894. [Google Scholar] [CrossRef]
- Yokelson, H.B.; Maxka, J.; Siegel, D.A.; West, R. 29Si NMR Observation of an Unprecedented Rearrangement in Tetraaryldisilenes. J. Am. Chem. Soc. 1986, 108, 4239–4241. [Google Scholar] [CrossRef]
- Shepherd, B.D.; Powell, D.R.; West, R. Synthesis, Geometrical Isomerism, and Crystal Structure of a Highly Hindered Disilene. Organometallics 1989, 8, 2664–2669. [Google Scholar] [CrossRef]
- Shizuka, H.; Sato, Y.; Ueki, Y.; Ishikawa, M.; Kumada, M. The 2pπ-3dπ Interaction in Aromatic Silanes. J. Chem. Soc. Faraday Trans. 1 1984, 80, 341–357. [Google Scholar] [CrossRef]
- Sakurai, H. Structure and reaction controlled by strong sigma/pi interactions. Pure Appl. Chem. 1987, 59, 1637–1646. [Google Scholar] [CrossRef]
- Yoon, U.C.; Mariano, P.S. Mechanistic and Synthetic Aspects of Amine-Enone Single Electron Transfer Photochemistry. Acc. Chem. Res. 1992, 25, 233–240. [Google Scholar] [CrossRef]
- Mizuno, K.; Tamai, T.; Sugimoto, A.; Maeda, H. Photoinduced Electron Transfer Reactions of Organosilicon Compounds. In Advances in Electron Transfer Chemistry; Mariano, P.S., Ed.; JAI Press: London, UK, 1999; Volume 6, pp. 131–165. [Google Scholar]
- Tamai, T.; Hayamizu, T.; Mizuno, K. Photoinduced Electron Transfer Reactions of Organosilicon Compounds. In Handbook of Photochemistry and Photobiology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2003; pp. 125–156. [Google Scholar]
- Mizuno, K.; Hayamizu, T.; Maeda, H. Regio- and stereoselective functionalization of electron-deficient alkenes by use of organosilicon compounds via photoinduced electron transfer. Pure Appl. Chem. 2003, 75, 1049–1054. [Google Scholar] [CrossRef]
- Daney, M.; Vanucci, C.; Desvergne, J.-P.; Castellan, A.; Bouas-Laurent, H. Photochemistry of Bichromophoric Systems. Silicon Guided Intramolecular 9,10:1’,2’ Anthracene Photodimerization in Bis(9-anthryl)dimethylsilane. Tetrahedron Lett. 1985, 26, 1505–1508. [Google Scholar] [CrossRef]
- Desvergne, J.-P.; Bitit, N.; Castellan, A.; Webb, M.; Bouas-Laurent, H. Non-conjugated Bichromophoric Systems. Part 4. Synthesis and Photochemical Study of Bis-9-anthryls with a Four-membered Chain; Influence of the Replacement of Methylene Links by Oxygen Atoms or Dimethylsilyl Groups on the Formation of Intramolecular Excimers and Photocyclomers. J. Chem. Soc. Perkin Trans. 2 1988, 1885–1894. [Google Scholar]
- Declercq, D.; De Schryver, F.C.; Miller, R.D. Fluorescence behaviour of pyrene substituted tri- and hexa-silanes. Chem. Phys. Lett. 1991, 186, 467–473. [Google Scholar] [CrossRef]
- Declercq, D.; Delbeke, P.; De Schryver, F.C.; Van Meervelt, L.; Miller, R.D. Ground- and Excited-State Interactions in Di-1-pyrenyl-Substituted Oligosilanes. J. Am. Chem. Soc. 1993, 115, 5702–5708. [Google Scholar] [CrossRef]
- Suzuki, H.; Satoh, S.; Kimata, Y.; Kuriyama, A. Synthesis and Properties of Poly(methylphenylsilane) Containing Anthracene Units. Chem. Lett. 1995, 451–452. [Google Scholar] [CrossRef]
- Kyushin, S.; Ikarugi, M.; Goto, M.; Hiratsuka, H.; Matsumoto, H. Synthesis and Electronic Properties of 9,10-Didilylanthracenes. Organometallics 1996, 15, 1067–1070. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Akiyama, S.; Tamao, K. Synthesis, Structures, Photophysical Properties, and Dynamic Stereochemistry of Tri-9-anthrylsilane Derivatives. Organometallics 1998, 17, 4347–4352. [Google Scholar] [CrossRef]
- Desvergne, J.-P.; Bonneau, R.; Dörr, G.; Bouas-Laurent, H. Photoprotodesilylation of 9-trimethylsilylanthracene in alcohols. Photochem. Photobiol. Sci. 2003, 2, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kyushin, S.; Takemasa, N.; Matsumoto, H.; Horiuchi, H.; Hiratsuka, H. 2,3,6,7,10,11-Hexakis(dimethylsilyl)triphenylene. Chem. Lett. 2003, 32, 1048–1049. [Google Scholar] [CrossRef]
- Karatsu, T.; Hazuku, R.; Nishioka, D.; Yagai, S.; Kobayashi, N.; Kitamura, A. Photoluminescence and Electroluminescence of 9,10-Bis(silylethynyl)anthracene. J. Photopolym. Sci. Tech. 2005, 18, 65–68. [Google Scholar]
- Kyushin, S.; Ishikita, Y.; Matsumoto, H.; Horiuchi, H.; Hiratsuka, H. Yellow-green Fluorescence of 5,11- and 5,12-Bis(diisopropylsilyl)naphthacenes. Chem. Lett. 2006, 35, 64–65. [Google Scholar] [CrossRef]
- Kyushin, S.; Matsuura, T.; Matsumoto, H. 2,3,4,5-Tetrakis(dimethylsilyl)thiophene: The First 2,3,4,5-Tetrasilylthiophene. Organometallics 2006, 25, 2761–2765. [Google Scholar] [CrossRef]
- Bénard, C.P.; Geng, Z.; Heuft, M.A.; VanCrey, K.; Fallis, A.G. Double Diels-Alder Strategies to Soluble 2,9- and 2,9,6,13-Tetraethynylpentacenes, Photolytic [4 + 4] Cycloadditions, and Pentacene Crystal Packing. J. Org. Chem. 2007, 72, 7229–7236. [Google Scholar] [CrossRef] [PubMed]
- Karatsu, T.; Hazuku, R.; Asuke, M.; Nishigaki, A.; Yagai, S.; Suzuri, Y.; Kita, H.; Kitamura, A. Blue electroluminescence of silyl substituted anthracene derivatives. Org. Electronics 2007, 8, 357–366. [Google Scholar] [CrossRef]
- Asai, K.; Konishi, G.; Nakajima, Y.; Kawauchi, S.; Ozawa, F.; Mizuno, K. Enhanced absorption and fluorescence efficiency of silylethynyl-functionalized oligothiophenes and thieno[3,2-b]thiophene. J. Organomet. Chem. 2011, 696, 1266–1271. [Google Scholar] [CrossRef]
- Maeda, H.; Inoue, Y.; Ishida, H.; Mizuno, K. UV Absorption and Fluorescence Properties of Pyrene Derivatives Having Trimethylsilyl, Trimethylgermyl, and Trimethylstannyl Groups. Chem. Lett. 2001, 1224–1225. [Google Scholar] [CrossRef]
- Maeda, H.; Maeda, T.; Mizuno, K.; Fujimoto, K.; Shimizu, H.; Inouye, M. Alkynylpyrenes as Improved Pyrene-Based Biomolecular Probes with the Advantages of High Fluorescence Quantum Yields and Long Absorption/Emission Wavelengths. Chem. Eur. J. 2006, 12, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ara, A.M.; Iimori, T.; Nakabayashi, T.; Maeda, H.; Mizuno, K.; Ohta, N. Electric Field Effects on Absorption and Fluorescence Spectra of Trimethylsilyl- and Trimethylsilylethynyl-Substituted Compounds of Pyrene in a PMMA Film. J. Phys. Chem. B 2007, 111, 10687–10696. [Google Scholar] [CrossRef] [PubMed]
- Tamai, T.; Watanabe, M.; Maeda, H.; Mizuno, K. Fluorescence Polymer Particles Incorporating Pyrene Derivatives. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1470–1475. [Google Scholar] [CrossRef]
- Maeda, H.; Ishida, H.; Inoue, Y.; Merpuge, A.; Maeda, T.; Mizuno, K. UV absorption and fluorescence properties of fused aromatic hydrocarbons having trimethylsilyl, trimethylgermyl, and trimethylstannyl groups. Res. Chem. Intermed. 2009, 35, 939–948. [Google Scholar] [CrossRef]
- Pitt, C.G.; Carey, R.N.; Toren, E.C., Jr. Nature of the Electronic Interactions in Aryl-Substituted Polysilanes. J. Am. Chem. Soc. 1972, 94, 3806–3811. [Google Scholar] [CrossRef]
- Ishikawa, M.; Oda, M.; Miyoshi, N.; Fábry, L.; Kumada, M.; Yamabe, T.; Akagi, K.; Fukui, K. Photochemically Generated Silicon-Carbon Double-Bonded Intermediates. 10. Photochemical Behavior of 1-Disilanyl- and 2-Disilanylnaphthalenes. J. Am. Chem. Soc. 1979, 101, 4612–4618. [Google Scholar] [CrossRef]
- Shizuka, H.; Obuchi, H.; Ishikawa, M.; Kumada, M. Photochemical and Photophysical Behaviour of Phenylsilanes and Naphthylsilanes. J. Chem. Soc. Faraday Trans. 1 1984, 80, 383–401. [Google Scholar] [CrossRef]
- Ohshita, J.; Ohsaki, H.; Ishikawa, M.; Tachibana, A.; Kurosaki, Y.; Yamabe, T.; Minato, A. Silicon-Carbon Unsaturated Compounds. 26. Photochemical Behavior of 1,4- and 1,5-Bis(pentamethyldisilanyl)naphthalene. Organometallics 1991, 10, 880–887. [Google Scholar] [CrossRef]
- Ohshita, J.; Ohsaki, H.; Ishikawa, M.; Tachibana, A.; Kurosaki, Y.; Yamabe, T.; Tsukihara, T.; Takahashi, K.; Kiso, Y. Silicon-Carbon Unsaturated Compounds. 29. Photochemical Behavior of 2,6- and 2,7-Bis(pentamethyldisilanyl)naphthalene. Organometallics 1991, 10, 2685–2695. [Google Scholar] [CrossRef]
- Karatsu, T.; Shibata, T.; Nishigaki, A.; Fukui, K.; Kitamura, A. π-π and σ-π Interactions in α,ω-Dinaphthyl and -Dianthryl Oligosilanes in Solution. Chem. Lett. 2001, 994–995. [Google Scholar] [CrossRef]
- Karatsu, T.; Shibata, T.; Nishigaki, A.; Kitamura, A.; Hatanaka, Y.; Nishimura, Y.; Sato, S.; Yamazaki, I. π-π and σ-π Interactions in α,ω-Di-(9-anthryl) and Di-(1-naphthyl) Oligosilanes Studied by Time-Resolved Fluorescence in Solution. J. Phys. Chem. B 2003, 107, 12184–12191. [Google Scholar] [CrossRef]
- Karatsu, T.; Terasawa, M.; Yagai, S.; Kitamura, A.; Nakamura, T.; Nishimura, Y.; Yamazaki, I. Time-resolved fluorescence of α-(9-anthryl)-ω-(1-naphthyl)-oligosilanes: Intramolecular electronic energy and charge transfer through π-π and σ-π interactions. J. Organomet. Chem. 2004, 689, 1029–1035. [Google Scholar] [CrossRef]
- Gerson, F.; Heinzer, J.; Bock, H.; Alt, H.; Seidl, H. ESR.-Messungen an Radikal-Anionen Trimethylsilyl-substituierter π-Elektronensysteme. Helv. Chim. Acta 1968, 51, 707–718. [Google Scholar] [CrossRef]
- Allred, A.L.; Bush, L.W. Electron Spin Resonance Studies of Silicon- and Germanium-Substituted Anion Radicals. J. Am. Chem. Soc. 1968, 90, 3352–3360. [Google Scholar] [CrossRef]
- Evans, A.G.; Jerome, B.; Rees, N.H. The dπ-pπ interaction in trimethylsilyl substituted naphthalenes. I. Study using polarography, andcharge transfer and ultraviolet spectroscopy. J. Chem. Soc. Perkin Trans. 2 1973, 447–452. [Google Scholar] [CrossRef]
- Evans, A.G.; Jerome, B.; Rees, N.H. dπ-pπ Interaction in Trimethylsilyl-substituted Naphthalenes. Part II. Electron Spin Resonance Studies. J. Chem. Soc. Perkin Trans. 2 1973, 2091–2093. [Google Scholar] [CrossRef]
- Sipe, H.J., Jr.; West, R. Electron Spin Resonance Studies of Group IV Organometallic Radical Anions. J. Organomet. Chem. 1974, 70, 353–366. [Google Scholar] [CrossRef]
- Kaim, W.; Tesmann, H.; Bock, H. Me3C-, Me3Si-, Me3Ge-, Me3Sn- und Me3Pb-substituierte Benzol- und Naphthalin-Derivate und ihre Radikalanionen. Chem. Ber. 1980, 113, 3221–3234. [Google Scholar] [CrossRef]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules, 2nd ed.; Academic Press, Inc.: New York, NY, USA, 1971. [Google Scholar]
- Eriksen, J.; Foote, C.S. Electron-Transfer Fluorescence Quenching and Exciplexes of Cyano-Substituted Anthracenes. J. Phys. Chem. 1978, 82, 2659–2662. [Google Scholar] [CrossRef]
- Mizuno, K.; Pac, C.; Sakurai, H. Photochemical Cyanation of Aromatic Hydrocarbons with Cyanide Anion. J. Chem. Soc. Chem. Commun. 1975, 553. [Google Scholar] [CrossRef]
- Yasuda, M.; Pac, C.; Sakurai, H. Photochemical Reactions of Aromatic Compounds. Part 34. Direct Photocyanation of Arenes with Sodium Cyanide in the Presence of Electron Acceptors. J. Chem. Soc. Perkin Trans. 1 1981, 746–750. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |











| R1 | R2 | R3 | Absorption a | Eigenvalues b (eV) | fluorescence | Quenching e | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) | log ε | HOMO | LUMO | energy gap | λema (nm) | τs c (aerated) (ns) | τs d (degassed) (ns) | Φf | kq (M−1s−1) | |||||||
| 1 | H | H | H | 286 | 3.58 | –8.765 | –0.467 | 8.298 | 324 | 16 | 96f | 0.23f | 2.69 × 109 | |||
| 2 | SiMe3 | H | H | 294 | 3.73 | –8.543 | –0.345 | 8.198 | 328 | 15 | 64 | 0.30g | 1.49 × 109 | |||
| 3 | SiMe2H | H | H | 294 | 3.75 | –8.568 | –0.362 | 8.206 | 328 | 15 | 61 | –i | 2.18 × 109 | |||
| 4 | SiMe2nBu | H | H | 294 | 3.74 | –8.560 | –0.361 | 8.199 | 329 | 15 | 63 | –i | –i | |||
| 5 | SiMe2tBu | H | H | 295 | 3.78 | –8.557 | –0.361 | 8.196 | 329 | 15 | 56 | –i | –i | |||
| 6 | SiMe2nOct | H | H | 294 | 3.76 | –8.562 | –0.363 | 8.199 | 329 | 16 | 62 | –i | 1.24 × 109 | |||
| 7 | SiMe3 | SiMe3 | H | 300 | 3.87 | –8.341 | –0.250 | 8.091 | 333 | 14 | 23 | 0.33g | –i | |||
| 8 | SiMe2H | SiMe2H | H | 300 | 3.86 | –8.385 | –0.280 | 8.105 | 333 | 13 | 30 | –i | 1.06 × 109 | |||
| 9 | SiMe2tBu | SiMe2tBu | H | 302 | 3.91 | –8.369 | –0.286 | 8.083 | 335 | 12 | –i | –i | –i | |||
| 10 | SiMe3 | OMe | H | 312 | 3.71 | –8.260 | –0.260 | 8.000 | 327 | 6 | 10 | 0.65g | –i | |||
| 11 | SiMe3 | CN | H | 315 | 3.88 | –8.876 | –0.956 | 7.920 | 333 | 8 | 11 | 0.66g | –i | |||
| 12 | SiMe3 | H | CN | 311 | 3.82 | –8.894 | –0.959 | 7.935 | 333 | 8 | –i | –i | –i | |||
| 13 | C≡CSiMe3 | H | H | 316 | 4.13 | –8.487 | –0.584 | 7.903 | 336 | 9 | –i | –i | –i | |||
| 14 | C≡CSiMe3 | C≡CSiMe3 | H | 347 | 4.65 | –8.236 | –0.714 | 7.522 | 352 | 2 | 2 | 0.85h | –i | |||
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maeda, H.; Maeda, T.; Mizuno, K. Absorption and Fluorescence Spectroscopic Properties of 1- and 1,4-Silyl-Substituted Naphthalene Derivatives. Molecules 2012, 17, 5108-5125. https://doi.org/10.3390/molecules17055108
Maeda H, Maeda T, Mizuno K. Absorption and Fluorescence Spectroscopic Properties of 1- and 1,4-Silyl-Substituted Naphthalene Derivatives. Molecules. 2012; 17(5):5108-5125. https://doi.org/10.3390/molecules17055108
Chicago/Turabian StyleMaeda, Hajime, Tomohiro Maeda, and Kazuhiko Mizuno. 2012. "Absorption and Fluorescence Spectroscopic Properties of 1- and 1,4-Silyl-Substituted Naphthalene Derivatives" Molecules 17, no. 5: 5108-5125. https://doi.org/10.3390/molecules17055108