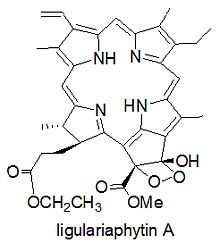
Phaeophytin Analogues from Ligularia knorringiana
Abstract
:1. Introduction
2. Results and Discussion

| Pos. | δH (J) | δC | Pos. | δH (J) | δC | Pos. | δH (J) | δC | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 141.4 s | 15 | 134.6 s | 121 | 3.90 (3H, s) | 12.3 q | ||||
| 2 | 131.6 s | 16 | 166.4 s | 131 | 102.1 s | |||||
| 3 | 136.2 s | 17 | 4.09 (1H, d, 9.2) | 53.8 d | 132 | 100.6 s | ||||
| 4 | 136.3 s | 18 | 4.47 (1H, q, 7.6) | 50.3 d | 133 | 171.1 s | ||||
| 5 | 9.54 (1H, s) | 99.8 d | 19 | 171.3 s | 134 | 3.77 (3H, s) | 54.4 q | |||
| 6 | 155.9 s | 20 | 8.72 (1H, s) | 94.0 d | 171 | 1.87 (1H, m) | 31.4 t | |||
| 7 | 136.7 s | 21 | 3.45 (3H, s) | 12.3 q | 2.45 (1H, m) | |||||
| 8 | 145.7 s | 31 | 8.03 (1H, dd, 18.0, 12.0) | 129.1 d | 172 | 2.18 (1H, m) | 32.3 t | |||
| 9 | 150.2 s | 32 | 6.17 (1H, br d, 11.2) | 122.9 t | 2.55 (1H, m) | |||||
| 10 | 9.76 (1H, s) | 104.3 d | 6.32 (1H, br d, 18.4) | 173 | 173.5 s | |||||
| 11 | 138.9 s | 71 | 3.27 (3H, s) | 11.5 q | 174 | 3.97 (2H, m) | 60.6 t | |||
| 12 | 131.7 s | 81 | 3.74 (2H, m) | 19.7 t | 175 | 1.07 (3H, t, 7.2) | 14.2 q | |||
| 13 | 111.3 s | 82 | 1.72 (3H, t, 7.6) | 17.8 q | 181 | 1.62 (3H, d, 7.6) | 22.4 q | |||
| 14 | 161.2 s |

3. Experimental
3.1. Plant Material
3.2. General
3.3. Extraction and Isolation
 +862 (c 0.06, CHCl3); IR νmax 3342, 2964, 2869, 1733, 1702, 1622, 1452 cm−1; UV (CHCl3): 670 (2.55), 614 (0.43), 531 (0.577), 500 (0.705), 402 (3.35) nm; 1H-NMR (CDCl3, 400 MHz) and 13C-NMR (CDCl3, 100 MHz) data see Table 1; TOF-MS: 653.2969 ([M+H]+, C37H40N4O7+; calc. 653.2975).
+862 (c 0.06, CHCl3); IR νmax 3342, 2964, 2869, 1733, 1702, 1622, 1452 cm−1; UV (CHCl3): 670 (2.55), 614 (0.43), 531 (0.577), 500 (0.705), 402 (3.35) nm; 1H-NMR (CDCl3, 400 MHz) and 13C-NMR (CDCl3, 100 MHz) data see Table 1; TOF-MS: 653.2969 ([M+H]+, C37H40N4O7+; calc. 653.2975).4. Conclusions
Supplementary Materials
Acknowledgements
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
References and notes
- Liu, S.W. Flora Republicae Popularis Sinicae; Science Press: Beijing, China, 1989; Volume 77, pp. 4–115. [Google Scholar]
- Yang, J.L.; Wang, R.; Shi, Y.P. Phytochemicals and biological activities of Ligularia species. Nat. Prod. Bioprospect. 2011, 1, 1–24. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, T.; Bastow, F.K.; Lee, K.H.; Chen, D.F. Altaicalarins A–D, cytotoxic bisabolane sesquiterpenes from Ligularia altaica. J. Nat. Prod. 2010, 73, 139–142. [Google Scholar] [CrossRef]
- Wu, Q.X.; Shi, Y.P.; Yang, Y. Unusual sesquiterpene lactones from Ligularia virgaurea spp. Oligocephala. Org. Lett. 2004, 6, 2313–2316. [Google Scholar] [CrossRef]
- Tori, M.; Watanabe, A.; Matsuo, S.; Okamoto, Y.; Tachikawa, K.; Takaoka, S.; Gong, X.; Kuroda, C.; Hanai, R. Diversity of Ligularia kanaitzensis in sesquiterpenoid composition and neutral DNA sequences. Tetrahedron 2008, 64, 4486–4495. [Google Scholar] [CrossRef]
- Saito, Y.; Hattori, M.; Iwamoto, Y.; Takashima, Y.; Mihara, K.; Sasaki, Y.; Fujiwara, M.; Sakaoku, M.; Shimizu, A.; Chao, X.; et al. Overlapping chemical and genetic diversity in Ligularia lamarum and Ligularia subspicata. Isolation of ten new eremophilanes and a new seco-bakkane compound. Tetrahedron 2011, 67, 2220–2231. [Google Scholar]
- Chee, C.F.; Lee, H.B.; Ong, H.C.; Ho, A.S.H. Photocytotoxic pheophorbide-related compounds from Aglaonema simplex. Chem. Biodivers. 2005, 2, 1648–1655. [Google Scholar] [CrossRef]
- Jin, P.F.; Deng, Z.W.; Pei, Y.H.; Lin, W.H. Two phaeophytin type analogues from marine sponge Dysidea sp. Chin. Chem. Lett. 2005, 16, 209–211. [Google Scholar]
- Buchanan, M.S.; Hashimoto, T.; Asakawa, Y. Phytyl esters and phaeophytins from the hornwort Megaceros flagellaris. Phytochemistry 1996, 41, 1373–1376. [Google Scholar]
- Schwikkard, S.L.; Mulholland, D.A.; Hutchings, A. Phaeophytins from Tapura fischeri. Phytochemistry 1998, 49, 2391–2394. [Google Scholar]
- Lee, T.H.; Lu, C.K.; Kuo, Y.H.; Jir, M.L.; Lo, C.K. Unexpected novel pheophytin peroxides from the leaves of Biden pilosa. Helv. Chim. Acta 2008, 91, 79–84. [Google Scholar] [CrossRef]
- Huang, X.P.; Li, M.; Xu, B.; Zhu, X.B.; Deng, Z.W.; Lin, W.H. Proteasome and NF-kB inhibiting phaeophytins from the green alga Cladophora fascicularis. Molecules 2007, 12, 582–592. [Google Scholar] [CrossRef]
- Sakata, K.; Yamamoto, K.; Ishikawa, H.; Yagi, A.; Etoh, H.; Ina, K. Chlorophyllone-a, a new pheophorbide-a related compound isolated from Ruditapes philippinarum as an antioxidative compound. Tetrahedron Lett. 1990, 31, 1165–1168. [Google Scholar]
- Cheng, H.H.; Wang, H.K.; Ito, J.; Bastow, K.F.; Tachibana, Y.; Nakanishi, Y.; Xu, Z.H.; Luo, T.Y.; Lee, K.H. Cytotoxic pheophorbide-related compounds from Clerodendrum calamitosum and C. crytophyllum. J. Nat. Prod. 2001, 64, 915–919. [Google Scholar] [CrossRef]
- Sgouras, D.; Duncan, R. Methods for the evaluation of biocompatibility of soluble synthetic polymers which have potential for biomedical use: 1–Use of the tetrazolium-based colorimetric assay (MTT) as a preliminary screen for evaluation of in vitro cyctotoxicity. J. Mater. Sci. Mater. Med. 1990, 1, 61–68. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.; Li, L.; Zheng, Q.; Kuroda, C.; Wang, Q. Phaeophytin Analogues from Ligularia knorringiana. Molecules 2012, 17, 5219-5224. https://doi.org/10.3390/molecules17055219
Li H, Li L, Zheng Q, Kuroda C, Wang Q. Phaeophytin Analogues from Ligularia knorringiana. Molecules. 2012; 17(5):5219-5224. https://doi.org/10.3390/molecules17055219
Chicago/Turabian StyleLi, Hui, Lina Li, Qiusheng Zheng, Chiaki Kuroda, and Qi Wang. 2012. "Phaeophytin Analogues from Ligularia knorringiana" Molecules 17, no. 5: 5219-5224. https://doi.org/10.3390/molecules17055219