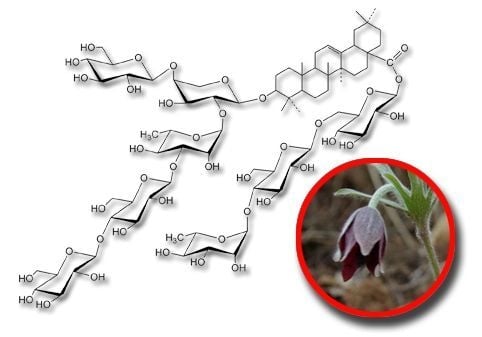
Saponins with Neuroprotective Effects from the Roots of Pulsatilla cernua
Abstract
:1. Introduction

2. Results and Discussion
| 1 | 4 | 1 | 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | ||
| C3- | glc″″-1 | 104.8 | 5.15(1H,d,7.8) | ||||||
| ara-1 | 105.1 | 4.61(1H,d,6.0) | 104.7 | 4.91(1H,d,6.0) | 2 | 74.5 | 3.95(1H,m) | ||
| 2 | 75.7 | 4.15(1H,m) | 75.5 | 4.42(1H,m) | 3 | 78.0 | 4.20(1H,m) | ||
| 3 | 73.8 | 4.33(1H,m) | 74.1 | 4.55(1H,m) | 4 | 71.2 | 4.89(1H,brs) | ||
| 4 | 79.1 | 4.74(1H,dd,2.4,9.6) | 80.7 | 4.30(1H,m) | 5 | 78.2 | 4.10(1H,m) | ||
| 5 | 65.1 | 3.69(1H,d,11), 4.40(1H,m) | 66.4 | 3.62(1H,d,6.0), 4.20(1H,m) | 6 | 62.2 | 4.27(1H,m), 4.53(1H,m) | ||
| glc-1 | 106.4 | 5.10(1H,d,7.8) | 105.0 | 4.99(1H,d,7.8) | C28- | ||||
| 2 | 75.3 | 4.15(1H,m) | 74.6 | 4.02(1H,m) | glc″-1 | 95.4 | 6.22(1H,d,7.8) | 95.8 | 6.14(1H,d,7.8) |
| 3 | 78.2 | 4.19(1H,m) | 78.6 | 4.20(1H,m) | 2 | 74.3 | 4.15(1H,m) | 74.2 | 4.15(1H,m) |
| 4 | 71.0 | 4.85(1H,m) | 71.6 | 4.20(1H,m) | 3 | 78.5 | 4.40(1H,m) | 78.9 | 4.40(1H,m) |
| 5 | 78.5 | 3.90(1H,m) | 78.4 | 3.90(1H,m) | 4 | 70.6 | 4.20(1H,m) | 71.0 | 4.33(1H,m) |
| 6 | 62.3 | 4.37(1H,m), 4.53(1H,m) | 62.5 | 4.25(1H,m), 4.45(1H,m) | 5 | 77.8 | 3.90(1H,m) | 78.4 | 3.92(1H,m) |
| rha-1 | 101.3 | 6.19(1H,brs) | 101.3 | 6.13(1H,brs) | 6 | 69.0 | 4.34(1H,m), 4.63(1H,m) | 69.4 | 4.33(1H,m), 4.82(1H,m) |
| 2 | 71.4 | 4.90(1H,m) | 72.0 | 4.65(1H,m) | glc″′-1 | 104.6 | 4.96(1H,d,7.2) | 103.1 | 4.90(1H,d,7.8) |
| 3 | 83.0 | 4.74(1H,dd,2.4,9.6) | 83.8 | 4.69(1H,dd,6.0,12.0) | 2 | 75.1 | 3.95(1H,m) | 74.9 | 3.93(1H,m) |
| 4 | 72.7 | 4.50(1H,m) | 73.2 | 4.35(1H,m) | 3 | 76.5 | 4.15(1H,m) | 76.7 | 4.13(1H,m) |
| 5 | 69.5 | 4.95(1H,m) | 69.6 | 4.65(1H,m) | 4 | 78.0 | 4.10(1H,m) | 78.2 | 4.09(1H,m) |
| 6 | 18.3 | 1.52(3H,d,6.0) | 18.6 | 1.50(3H,d,6.0) | 5 | 76.9 | 3.61(1H,d,9.0) | 77.3 | 3.57(1H,d,9.0) |
| glc′-1 | 106.3 | 5.42(1H,d,7.8) | 106.7 | 5.11(1H,d,7.8) | 6 | 61.0 | 4.05(1H,m), 4.17(1H,m) | 61.5 | 4.05(1H,m), 4.15(1H,m) |
| 2 | 75.2 | 3.95(1H,m) | 75.8 | 3.90(1H,m) | rha′-1 | 102.5 | 5.84(1H,brs) | 102.4 | 5.76(1H,brs) |
| 3 | 76.5 | 4.30(1H,m) | 76.7 | 4.30(1H,m) | 2 | 72.3 | 4.69(1H,m) | 72.7 | 4.65(1H,m) |
| 4 | 81.0 | 4.35(1H,m) | 69.7 | 4.40(1H,m) | 3 | 72.5 | 4.50(1H,m) | 72.9 | 4.55(1H,m) |
| 5 | 76.3 | 4.10(1H,m) | 77.0 | 4.10(1H,m) | 4 | 73.7 | 4.30(1H,m) | 74.1 | 4.35(1H,m) |
| 6 | 61.7 | 3.84(1H,m), 4.43(1H,m) | 62.0 | 3.84(1H,m), 4.43(1H,m) | 5 | 70.1 | 4.31(1H,m) | 70.5 | 4.93(1H,m) |
| 6 | 18.3 | 1.66(3H,d,6.0) | 18.7 | 1.61(3H,d,6.0) | |||||
| 2 | 3 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | ||
| C3- | glc′-1 | 106.4 | 5.40(1H,d,7.8) | 106.7 | 5.39(1H, O) | ||||
| ara-1 | 105.2 | 4.76(1H,d,6.0) | 104.9 | 4.95(1H,d,6.0) | 2 | 75.4 | 3.90(1H,m) | 75.4 | 3.90(1H,m) |
| 2 | 75.2 | 4.49(1H,m) | 75.3 | 4.49(1H,m) | 3 | 78.2 | 4.19(1H,m) | 78.2 | 4.20(1H,m) |
| 3 | 74.0 | 4.29(1H,m) | 74.0 | 4.25(1H,m) | 4 | 71.7 | 4.85(1H,m) | 71.8 | 4.25(1H,m) |
| 4 | 81.9 | 4.22(1H,m) | 82.0 | 4.19(1H,m) | 5 | 78.2 | 4.15(1H,m) | 78.2 | 4.15(1H,m) |
| 5 | 65.8 | 3.75(1H,d,30), 4.16(1H,m) | 66.6 | 3.60(1H,m), 4.18(1H,m) | 6 | 61.8 | 4.38(1H,m), 4.49(1H,m) | 61.8 | 4.36(1H,m), 4.42(1H,m) |
| glc-1 | 104.9 | 5.11(1H,d,7.2) | 105.1 | 5.06(1H,d,7.8) | C28- | ||||
| 2 | 73.9 | 4.10(1H,m) | 72.6 | 4.49(1H,m) | glc″-1 | 95.6 | 6.21(1H, O) | 95.7 | 6.20(1H,d,8.4) |
| 3 | 78.2 | 4.19(1H,m) | 78.2 | 4.15(1H,m) | 2 | 74.7 | 4.15(1H,m) | 74.9 | 4.15(1H,m) |
| 4 | 71.2 | 4.40(1H,m) | 71.0 | 4.25(1H,m) | 3 | 78.6 | 4.39(1H,m) | 78.8 | 4.45(1H,m) |
| 5 | 77.0 | 4.00(1H,m) | 76.8 | 4.00(1H,m) | 4 | 70.8 | 4.25(1H,m) | 70.8 | 4.25(1H,m) |
| 6 | 68.5 | 3.91(1H,m), 4.58(1H,m) | 68.6 | 3.90(1H,m), 4.57(1H,d,9.0) | 5 | 78.0 | 4.00(1H,m) | 78.2 | 3.90(1H,m) |
| rha″-1 | 102.7 | 5.39(1H,brs) | 102.8 | 5.37(1H,brs) | 6 | 69.1 | 4.32(1H,m), 4.60(1H,m) | 69.2 | 4.29(1H,m), 4.63(1H,m) |
| 2 | 71.8 | 3.90(1H,m) | 72.0 | 4.85(1H,m) | glc″′-1 | 104.8 | 4.96(1H, O) | 104.9 | 4.92(1H, O) |
| 3 | 71.8 | 4.85(1H,m) | 72.0 | 4.65(1H,m) | 2 | 75.2 | 3.95(1H,m) | 75.3 | 3.95(1H,m) |
| 4 | 73.9 | 4.29(1H,m) | 73.1 | 4.44(1H,m) | 3 | 76.4 | 4.13(1H,m) | 76.5 | 4.15(1H,m) |
| 5 | 69.8 | 4.25(1H,m) | 69.9 | 4.21(1H,m) | 4 | 78.1 | 4.10(1H,m) | 78.2 | 4.00(1H,m) |
| 6 | 18.4 | 1.49(3H,d,6.0) | 18.5 | 1.51(3H,d,6.0) | 5 | 77.1 | 3.61(1H,d,12) | 77.2 | 3.62(1H,m) |
| rha-1 | 101.4 | 6.23(1H,brs) | 101.4 | 6.23(1H,brs) | 6 | 61.2 | 4.05(1H,m), 4.17(1H,m) | 61.3 | 4.05(1H,m), 4.17(1H,m) |
| 2 | 71.6 | 4.25(1H,m) | 71.7 | 3.90(1H,m) | rha′-1 | 102.7 | 5.85(1H,brs) | 102.9 | 5.81(1H,brs) |
| 3 | 83.5 | 4.70(1H,m) | 83.5 | 4.75(1H,dd,3.0,9.6) | 2 | 72.5 | 4.63(1H,m) | 72.6 | 4.65(1H,m) |
| 4 | 73.0 | 4.43(1H,m) | 72.8 | 4.17(1H,m) | 3 | 72.7 | 4.49(1H,m) | 72.8 | 4.50(1H,m) |
| 5 | 69.6 | 4.59(1H,m) | 69.7 | 4.06(1H,m) | 4 | 73.8 | 4.30(1H,m) | 73.9 | 4.28(1H,m) |
| 6 | 18.5 | 1.54(3H,d,6.0) | 18.7 | 1.55(3H,d,6.0) | 5 | 70.2 | 4.90(1H,m) | 70.4 | 4.91(1H,m) |
| 6 | 18.4 | 1.67(3H,d,6.0) | 18.6 | 1.66(3H,d,6.0) | |||||


3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Acid Hydrolysis and GC Analysis
3.6. Cell Culture
3.7. Determination of Cell Viability
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
References and Notes
- Kang, S.S. Saponins from the roots of Pulsatilla koreana. Arch. Pharm. Res. 1989, 12, 42–47. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Ye, W.C.; Che, C.T.; Zhao, S.X. Triterpene saponins from Pulsatilla cernua. Acta Pharm. Sin. 2000, 35, 756–759. [Google Scholar]
- Zhang, Q.W.; Ye, W.C.; Yan, X.Z.; Zhu, G.; Che, C.T.; Zhao, S.X. Cernuosides A and B, two sucrase inhibitors from Pulsatilla cernua. J. Nat. Prod. 2000, 63, 276–278. [Google Scholar] [CrossRef]
- Bang, S.C.; Lee, J.H.; Song, G.Y.; Kim, D.H.; Yoon, M.Y.; Ahn, B.Z. Antitumor activity of Pulsatilla koreana saponins and their structure-activity relationship. Chem. Pharm. Bull. 2005, 53, 1451–1454. [Google Scholar] [CrossRef]
- Xu, T.H.; Xu, Y.J.; Han, D.; Zhao, H.F.; Xie, S.X.; Xu, D.M. Triterpenoid saponins from Pulsatilla cernua (Thunb.) Bercht. et Opiz. J. Integr. Plant Biol. 2006, 49, 202–206. [Google Scholar]
- Xu, T.H.; Xu, Y.J.; Li, H.X.; Han, D.; Zhao, H.F.; Xie, S.X.; Li, Y.; Niu, J.Z.; Si, Y.S.; Xu, D.M. Two new triterpenoid saponins from Pulsatilla cernua (Thunb.) Bercht. et Opiz. J. Asian Nat. Prod. Res. 2007, 9, 705–711. [Google Scholar] [CrossRef]
- Fu, Y.M.; Chen, H.; Liu, D.L.; Zhang, Z.M. Study on the chemical components of Pulsatilla cernua. Chin. Tradit. Herbal Drugs 2008, 39, 26–29. [Google Scholar]
- Xu, Y.J.; Bai, L.; Liu, Y.H.; Liu, Y.; Xu, T.H.; Xie, S.X.; Si, Y.S.; Zhou, H.O.; Liu, T.H.; Xu, D.M. A new triterpenoid saponin from Pulsatilla cernua. Molecules 2010, 15, 1891–1897. [Google Scholar] [CrossRef]
- Yang, H.; Cho, Y.W.; Kim, S.H.; Kim, Y.C.; Sung, S.H. Triterpenoidal saponins of Pulsatilla koreana roots. Phytochemistry 2010, 71, 1892–1899. [Google Scholar] [CrossRef]
- Seo, J.S.; Kim, T.K.; Lee, Y.H.; Lee, K.W.; Park, S.K.; Baek, I.S.; Kim, K.S.; Im, G.J.; Lee, S.M.; Park, Y.H.; Han, P.L. SK-PC-B70M confers anti-oxidant activity and reduces Aβ levels in the brain of Tg2576 mice. Brain Res. 2009, 1261, 100–108. [Google Scholar] [CrossRef]
- Han, C.K.; Choi, W.R.; Oh, K.B. Cognition-enhancing and neuroprotective effects of hederacolchiside E from Pulsatilla koreana. Planta. Med. 2007, 73, 665–669. [Google Scholar] [CrossRef]
- Dubois, M.A.; Ilyas, M.; Wagner, H. Cussonosides A and B, two triterpene-saponins from Cussonia barteri. Planta. Med. 1986, 52, 80–83. [Google Scholar] [CrossRef]
- Li, X.C.; Wang, D.Z.; Wu, S.G. Triterpenoid saponins from Pulsatilla campanella. Phytochemistry 1990, 29, 595–599. [Google Scholar]
- Saito, S.; Sumita, S.; Tamura, N.; Nagamura, Y.; Nishida, K.; Ito, M.; Ishiguro, I. Saponins from the leaves of Aralia elata Seem. (Araliaceae). Chem. Pharm. Bull. 1990, 38, 411–414. [Google Scholar] [CrossRef]
- Shi, B.J.; Li, Q.; Zhang, X.Q.; Wang, Y.; Ye, W.C.; Yao, X.S. Triterpene glycosides from the aerial parts of Pulsatilla chinensis. Acta Pharm. Sin. 2007, 42, 862–866. [Google Scholar]
- Mahato, S.B.; Kundu, A.P. 13CNMR spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar]
- Kim, H.J.; Lee, K.W.; Lee, H.J. Protective effects of piceatannol against beta-amyloid-induced neuronal cell death. Ann. N. Y. Acad. Sci. 2007, 1095, 473–482. [Google Scholar] [CrossRef]
- Nie, B.M.; Jiang, X.Y.; Cai, J.X.; Fu, S.L.; Yang, L.M.; Lin, L.; Hang, Q.; Lu, P.L.; Lu, Y. Panaxydol and panaxynol protect cultured cortical neurons against Abeta25-35-induced toxicity. Neuropharm. 2008, 54, 845–853. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.-Y.; Guan, Y.-L.; Zou, L.-B.; Gong, Y.-X.; Hua, H.-M.; Xu, Y.-N.; Zhang, H.; Yu, Z.-G.; Fan, W.-H. Saponins with Neuroprotective Effects from the Roots of Pulsatilla cernua. Molecules 2012, 17, 5520-5531. https://doi.org/10.3390/molecules17055520
Liu J-Y, Guan Y-L, Zou L-B, Gong Y-X, Hua H-M, Xu Y-N, Zhang H, Yu Z-G, Fan W-H. Saponins with Neuroprotective Effects from the Roots of Pulsatilla cernua. Molecules. 2012; 17(5):5520-5531. https://doi.org/10.3390/molecules17055520
Chicago/Turabian StyleLiu, Jian-Yu, Ying-Li Guan, Li-Bo Zou, Yi-Xia Gong, Hui-Ming Hua, Yong-Nan Xu, Hui Zhang, Zong-Gui Yu, and Wen-Hao Fan. 2012. "Saponins with Neuroprotective Effects from the Roots of Pulsatilla cernua" Molecules 17, no. 5: 5520-5531. https://doi.org/10.3390/molecules17055520