Metathesis Transformations of Natural Products: Cross-Metathesis of Natural Rubber and Mandarin Oil by Ru-Alkylidene Catalysts
Abstract
:1. Introduction
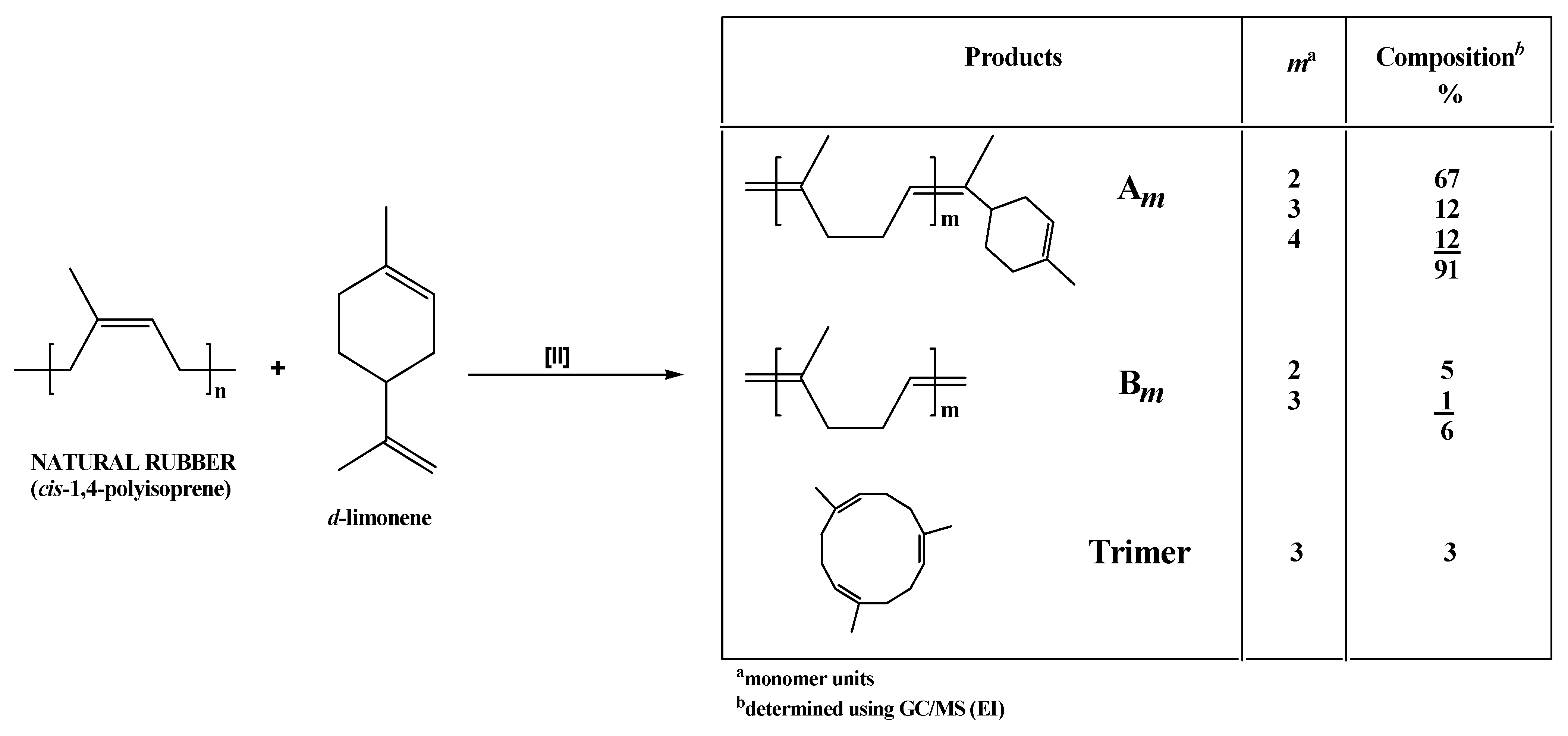
2. Results and Discussion
3. Experimental
3.1. Reagents
3.2. Techniques
3.3. Degradation Procedure in Organic Solvents
3.4. Procedure for the Metathesis Degradation of NR
4. Conclusions
Acknowledgements
References and Notes
- Bilel, H.; Hamdi, N.; Zagrouba, F.; Fischmeister, C.; Bruneau, Ch. Cross-metathesis transformations of terpenoids in dialkyl carbonate solvents. Green Chem. 2011, 13, 1448–1452. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Patel, J.; Mujcinovic, S.; Jackson, R.W.; Robinson, A.J. Preparation of terminal oxygenates from renewable natural oils by a one-pot metathesis-isomerization-methoxycarbonylation-transesterification reaction sequence. Green Chem. 2006, 8, 746–749. [Google Scholar] [CrossRef]
- Mol, J.C. Catalytic metathesis of unsaturated fatty acid esters and oils. Top. Catal. 2004, 27, 97–104. [Google Scholar] [CrossRef]
- Bruneau, C.; Fischmeiser, C.; Miao, X.; Malacea, R.; Dixneuf, P.H. Cross-metathesis with acrylonitrile and applications to fatty acid derivatives. Eur. J. Lipid Sci. Technol. 2010, 112, 3–9. [Google Scholar] [CrossRef]
- Rybak, A.; Meier, M.A.R. Cross-metathesis of fatty acid derivatives with methyl acrylate: renewable raw materials for the chemical industry. Green Chem. 2007, 9, 1356–1361. [Google Scholar] [CrossRef]
- Mathers, R.T.; McMahon, K.C.; Damodaran, K.; Retarides, C.J.; Kelly, D.J. Ring-opening metathesis polymerization in D-limonene: A renewable polymerization solvent and chain transfer agent for the synthesis of alkene macromonomers. Macromolecules 2006, 39, 8982–8986. [Google Scholar] [CrossRef]
- Mathers, R.T.; Damodaran, K.; Rendos, M.G.; Lavrich, M.S. Functional hyperbranched polymers using ring-opening metathesis polymerization of dicyclopentadiene with monoterpenes. Macromolecules 2009, 42, 1512–1518. [Google Scholar] [CrossRef]
- Gutierrez, S.; Tlenkopatchev, M.A. Metathesis of renewable products: Degradation of natural rubber via cross-metathesis with β-pinene using Ru-alkylidene catalysts. Polym. Bull. 2011, 66, 1029–1038. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hirasawa, H. Sequence analysis of polyprenols by 500 MHz 1H-NMR spectroscopy. Chem. Phys. Lipids 1989, 51, 183–189. [Google Scholar] [CrossRef]
- Baldenius, K.U.; Von Dem Bussche-Hünnefeld, L.; Hilgemann, E.; Hoppe, P.; Stürmer, R. Ullmanns Encyclopedia of Industrial Chemistry; VCH Verlagsgesellschaft: New York, NY, USA, 1966; Volume 7, pp. 478–488, 594–597. [Google Scholar]
- Stevens, P.M. Polymer Chemistry, 3rd ed.; Oxford University Press, Inc.: New York, NY, USA, 1999; pp. 35–37, 169–170, 252–254. [Google Scholar]
- Ivin, K.J.; Mol, J.C. Olefin Metathesis and Metathesis Polymerization; Academic Press: San Diego, CA, USA, 1997; Chapter 16; p. 375. [Google Scholar]
- Craig, S.W.; Manzer, J.A.; Coughlin, E.B. Highly efficient acyclic diene metathesis depolymerization using a ruthenium catalyst containing a N-heterocyclic carbene ligand. Macromolecules 2001, 39, 7929–7931. [Google Scholar] [CrossRef]
- Fomine, S.; Tlenkopatchev, M.A. Computational modeling of renewable molecules. Ruthenium alkylidene-mediated metathesis of trialkyl-substituted olefins. Organometallics 2010, 29, 1580–1587. [Google Scholar] [CrossRef]
- Fomine, S.; Tlenkopatchev, M.A. Metathesis transformations of terpenes. Computational modeling of (−)-α-pinene ring opening by ruthenium and tungsten carbene catalysts. J. Organomet. Chem. 2012, 701, 68–74. [Google Scholar] [CrossRef]
- Lota, M.L.; Rocca, S.D.; Tomi, F.; Casanova, J. Chemical variability of peel and leaf essential oils of 15 species of mandarins. Biochem. Syst. Ecol. 2001, 29, 77–104. [Google Scholar] [CrossRef]
- Martínez, A.; Tlenkopatchev, M.A. Metathesis degradation of natural rubber in the presence of avocado and mandarin oils using ruthenium alkylidene catalysts. In Proceedings of the European Polymer Congress EPF 2011 and XII Congress of the Specialized Group of Polymer GEP, Granada, Spain, 26 June–1 July 2011; p. 652. [Google Scholar]
- Alimuniar, A.; Yarmo, M.A.; Rahman, M.Z.; Kohjiya, S.; Ikeda, Y.; Yamashita, S. Metathesis degradation of natural rubber. Polym. Bull. 1990, 23, 119. [Google Scholar] [CrossRef]
- Gutierrez, S.; Vargas, S.M.; Tlenkopatchev, M.A. Computational study of metathesis degradation of rubber. Distributions of products for the ethenolysis of 1,4-polyisoprene. Polym. Degrad. Stabil. 2004, 83, 149–156. [Google Scholar] [CrossRef]
- Solanky, S.S.; Campistron, I.; Laguerre, A.; Pilard, J.-F. Metathetic selective degradation of polyisoprene: Low-molecular-weight telechelic oligomer obtained from both synthetic and natural rubber. Macromol. Chem. Phys. 2005, 206, 1057–1063. [Google Scholar] [CrossRef]
- Wolf, S.; Plenio, H. On ethenolysis of natural rubber and squalene. Green Chem. 2011, 13, 2008–2012. [Google Scholar] [CrossRef]
- Tlenkopatchev, M.A.; Barcenas, A.; Fomine, S. Computional study of metathesis degradation of rubber, 2. distribution of cyclic oligomers via intramolecular metathesis degradation of natural rubber. Macromol. Theor. Simul. 2001, 10, 441–446. [Google Scholar] [CrossRef]
- Thorn-Csanyi, E.; Hummer, J.; Zilles, J.U. Metathetic ring-chain equilibrium; synthesis of 1,5,9-trimethyl-(1E,5E,9E)-cyclododecatriene from 1,4-polyisoprene. Macromol. Rapid Commun. 1994, 15, 797–800. [Google Scholar] [CrossRef]
- GC-2010/MS-QP2010, version 2.5; Shimadzu GCMS solution software, Kyoto, Japan.
Sample Availability: Sample of the compounds are available from the author. |




| No. | COMPOUNDS | COMPOSITION % |
|---|---|---|
| 1 | d-limonene | 74.0 |
| 2 | α-pinene | 4.2 |
| 3 | β-pinene | 3.0 |
| 4 | p-cymene | 0.6 |
| 5 | γ-terpinene | 15.6 |
| 6 | β-myrcene | 1.3 |
| 7 | Dimethyl anthranilate | 0.5 |
| 8 | Geraniol acetate, citronellol | 0.8 |
| Entry | Catalyst | CTA | [NR] d:[CTA] | Time (h) | Temp (°C) | Yield e (%) | Mw f (theor) | Mn g (1H-NMR) | Mn h (GPC) | MWD h (GPC) |
|---|---|---|---|---|---|---|---|---|---|---|
| Natural rubber a | 1.7*106 | 1.50 | ||||||||
| 1 | II | d-limonene | 1:1 | 24 | 50 | 80 | 204 | 722 | 779 | 2.1 |
| 2 | II | d-limonene | 1:1 | 24 | 80 | 97 | 204 | 525 | 501 | 2.2 |
| 3 b | II | d-limonene | 1:10 | 24 | 50 | 81 | 204 | 771 | 764 | 2.0 |
| 4 b | II | Mandarin oil | 1:10 | 24 | 50 | 83 | 204 | 815 | 801 | 2.2 |
| 5 c | II | Mandarin oil | 1:1 | 24 | 50 | 82 | 204 | 827 | 861 | 2.2 |
| 6 | II | Mandarin oil | 1:1 | 2 | 50 | 87 | 204 | 11,745 | 16,724 | 2.2 |
| 7 | II | Mandarin oil | 1:1 | 8 | 50 | 92 | 204 | 8,216 | 13,452 | 2.2 |
| 8 | II | Mandarin oil | 1:1 | 12 | 50 | 92 | 204 | 4,216 | 6,678 | 2.1 |
| 9 | II | Mandarin oil | 1:1 | 24 | 50 | 80 | 204 | 836 | 811 | 2.1 |
| 10 | II | Mandarin oil | 1:1 | 48 | 50 | 82 | 204 | 796 | 779 | 2.0 |
| 11 | II | Mandarin oil | 5:1 | 24 | 50 | 95 | 476 | 3,184 | 4,745 | 2.2 |
| 12 | II | Mandarin oil | 10:1 | 24 | 50 | 92 | 816 | 5,674 | 7,281 | 2.1 |
| 13 | I | Mandarin oil | 1:1 | 48 | 50 | 70 | 204 | 39,000 | 41,242 | 1.8 |
| 14 | I | Mandarin oil | 1:1 | 48 | 80 | 72 | 204 | 16,900 | 17,667 | 1.8 |
| 15 | I | Mandarin oil | 1:1 | 48 | 100 | 74 | 204 | 16,836 | 17,554 | 2.0 |
© 2012 by the authors. icensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martínez, A.; Gutiérrez, S.; Tlenkopatchev, M.A. Metathesis Transformations of Natural Products: Cross-Metathesis of Natural Rubber and Mandarin Oil by Ru-Alkylidene Catalysts. Molecules 2012, 17, 6001-6010. https://doi.org/10.3390/molecules17056001
Martínez A, Gutiérrez S, Tlenkopatchev MA. Metathesis Transformations of Natural Products: Cross-Metathesis of Natural Rubber and Mandarin Oil by Ru-Alkylidene Catalysts. Molecules. 2012; 17(5):6001-6010. https://doi.org/10.3390/molecules17056001
Chicago/Turabian StyleMartínez, Araceli, Selena Gutiérrez, and Mikhail A. Tlenkopatchev. 2012. "Metathesis Transformations of Natural Products: Cross-Metathesis of Natural Rubber and Mandarin Oil by Ru-Alkylidene Catalysts" Molecules 17, no. 5: 6001-6010. https://doi.org/10.3390/molecules17056001




