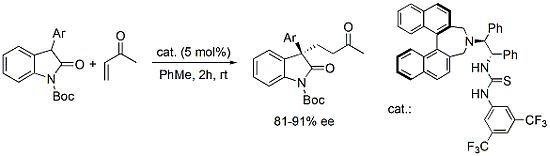
Enantioselective Michael Addition of 3-Aryl-Substituted Oxindoles to Methyl Vinyl Ketone Catalyzed by a Binaphthyl-Modified Bifunctional Organocatalyst
Abstract
:1. Introduction
2. Results and Discussion

| Entry | Cat. | Solvent | Time (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|
| 1 | I | PhMe | 3 | 73 | 5 |
| 2 | II | PhMe | 3 | 76 | 5 |
| 3 | III | PhMe | 2 | 82 | 25 |
| 4 | IV | PhMe | 5 | 83 | 15 |
| 5 | V | PhMe | 2 | 88 | 91 |
| 6 | VI | PhMe | 2 | 80 | 67 |
| 7 | III | CH2Cl2 | 2 | 90 | 83 |
| 8 | III | THF | 3 | 78 | 81 |
| 9 | III | Et2O | 3 | 86 | 73 |
| 10 | III | p-xylene | 4 | 78 | 87 |
| 11 | III | m-xylene | 4 | 81 | 85 |
| 12 c | V | PhMe | 5 | 83 | 87 |
| 13 d | V | PhMe | 5 | 70 | 59 |

| Entry | 1, Ar | Yield (%) a | ee (%) b |
|---|---|---|---|
| 1 | 1a, Ph | 88 | 3a, 91 |
| 2c | 1b, m-MeC6H4 | 91 | 3b, 83 |
| 3 | 1c, p-MeC6H4 | 88 | 3c, 81 |
| 4 | 1d, m-MeOC6H4 | 86 | 3d, 91 |
| 5 c | 1e, p-FC6H4 | 87 | 3e, 85 |
| 6 | 1f, 2-naphthyl | 82 | 3f, 83 |

3. Experimental
3.1. General
3.2. Typical Procedure for the Conjugate Addition Reaction of 3-Phenyloxindole (1a) with Methyl Vinyl Ketone (2a)
3.3. Typical Procedure for the Conjugate Addition Reaction of 3-Phenyloxindole (1a) with 1,1-Bis(benzenesulfonyl)ethylene (4a)
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 3 and 5 are available from the authors.
References and Notes
- Marti, C.; Carreira, E.M. Construction of spiro[pyrrolidine-3,3′-oxindoles]-Recent Applications to the Synthesis of Oxindole Alkaloids. Eur. J. Org. Chem. 2003, 2003, 2209–2219. [Google Scholar] [CrossRef]
- Dounay, A.B.; Overman, L.E. The Asymmetric Intramolecular Heck Reaction in Natural Product Total Synthesis. Chem. Rev. 2003, 103, 2945–2964. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-Spirooxindole Natural Prodcuts as Inspirations for the Develolpment of Potential Therapeutic Agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Trost, B.M.; Brennan, M.K. Asymmetric Syntheses of Oxindole and Indole Spirocyclic Alkaoid Natural Procducts. Synthesis 2009, 3003–2025. [Google Scholar]
- Zhou, F.; Liu, Y.-L.; Zhou, J. Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Adv. Synth. Catal. 2010, 352, 1381–1407. [Google Scholar] [CrossRef]
- Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Sodeoka, M. Catalytic Enantioselective Fluorinatioin of Oxindoles. J. Am. Chem. Soc. 2005, 127, 10164–10165. [Google Scholar] [CrossRef]
- Trost, B.M.; Frederiksen, M.U. Palladium-Catalyzed Asymmetric Allylation of Prochiral Nucleophiles: Synthesis of 3-Allyl-3-Aryl Oxindoles. Angew. Chem. Int. Ed. 2005, 44, 308–310. [Google Scholar] [CrossRef]
- Shintani, R.; Inoue, M.; Hayashi, T. Rhodium-Catalyzed Asymmetric Addtion of Aryl- and Alkenylboronic Acids to Isatins. Angew. Chem. Int. Ed. 2006, 45, 3353–3356. [Google Scholar] [CrossRef]
- Trost, B.M.; Zhang, Y. Mo-Catalyzed Regio- Diastereo-, and Enantioselective Allylic Alkylation of 3-Aryloxindoles. J. Am. Chem. Soc. 2007, 129, 14548–14549. [Google Scholar] [CrossRef]
- Corkey, B.K.; Toste, F.D. Palladium-Catalyzed Enantioselective Cyclization of Silyloxy-1,6-Enyens. J. Am. Chem. Soc. 2007, 129, 2764–2765. [Google Scholar] [CrossRef]
- Kundig, E.P.; Seidel, T.M.; Jia, Y.-X.; Bernardinelli, G. Bulky Chiral Carbene Ligands and Their Application in the Palladium-Catalyzed Asymmetric Intramolecular α-Arylation of Amides. Angew. Chem. Int. Ed. 2007, 46, 8484–8487. [Google Scholar] [CrossRef]
- Linton, E.C.; Kozlowski, M.C. Catalytic Enantioselective Meerwein-Eschenmoser Claisen Rearrangement: Asymmetric Synthesis of Allyl Oxindoles. J. Am. Chem. Soc. 2008, 130, 16162–16163. [Google Scholar] [CrossRef]
- Jia, Y.-X.; Hillgren, J.M.; Watson, E.L.; Marsden, S.P.; Kundig, E.P. Chiral N-heterocylic carbene ligands for asymmetric catalytic oxindole synthesis. Chem. Commun. 2008, 4040–4042. [Google Scholar]
- Kato, Y.; Furutachi, M.; Chen, Z.; Mitsunuma, H.; Matsunaga, S.; Shibasaki, M. A Homodinuclear Mn(III)2−Schiff Base Complex for Catalytic Asymmetric 1,4-Additions of Oxindoles to Nitroalkenes. J. Am. Chem. Soc. 2009, 131, 9168–9169. [Google Scholar]
- Tomita, D.; Yamatsugu, K.; Kanai, M.; Shibasaki, M. Enantioselective Synthesis of SM-130686 based on the Development of Asymmetric Cu(I)F Catalysis To Access 2-Oxindoles Containing a Tetrasubstituted Carbon. J. Am. Chem. Soc. 2009, 131, 6946–6948. [Google Scholar] [CrossRef]
- Trost, B.M.; Zhang, Y. Catalytic Double Stereoinduction in Asymmetric Allylic Alkylation of Oxindoles. Chem. Eur. J. 2010, 16, 296–303. [Google Scholar] [CrossRef]
- Trost, B.M.; Czabaninuk, L.C. Palladium-Catalyzed Asymmetric Benzylation of 3-Aryl Oxindoles. J. Am. Chem. Soc. 2010, 132, 15534–15536. [Google Scholar] [CrossRef]
- Mouri, S.; Chen, Z.; Mitsunuma, H.; Furutachi, M.; Matsunaga, S.; Shibasaki, M. Catalytic Asymmetric Synthesis of 3-Aminooxindoles: Enantiofacial Selectivity Switch in Bimetallic vs. Monometallic Schiff base Catalysis. J. Am. Chem. Soc. 2010, 132, 1255–1257. [Google Scholar]
- Bui, T.; Syed, S.; Barbas, C.F., III. Thiourea-Catalyzed Highly Enantio- and Diastereoselective Additions of Oxindoles to Nitroolefins: Application to the Formal Synthesis of (+)-Physostigmine. J. Am. Chem. Soc. 2009, 131, 8758–8759. [Google Scholar]
- Galzerano, P.; Bencivenni, G.; Pesciaioli, F.; Mazzanti, A.; Giannichi, B.; Sambri, L.; Bartoli, G.; Melchiorre, P. Asymmetric Iminium Ion Catalysis with a Novel Bifunctional Primary Amine Thiourea: Controlling Adjacent Quaternary and Teriary Stereocenters. Chem. Eur. J. 2009, 15, 7846–7849. [Google Scholar] [CrossRef]
- Bravo, N.; Mon, I.; Companyo, X.; Alba, A.-N.; Moyano, A.; Rios, R. Enantioselective addition of oxindoles to aliphatic α,β-unsaturated aldehydes. Tetrahedron Lett. 2009, 50, 6624–6626. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Xi, Z.-G.; Luo, S.; Cheng, J.-P. Asymmetric Michael Addition Reaction of 3-Substituted Oxindoles to Nitroolefins Catalyzed by a Chiral Alkyl- Substituted Thiourea Catalyst. Adv. Synth. Catal. 2010, 352, 416–242. [Google Scholar] [CrossRef]
- Zhu, Q.; Lu, Y. Stereocontrolled Creation of All-Carbon Quaternary Stereocenters by Organocatalytic Conjugate Addition of Oxindoles to Vinyl Sulfone. Angew. Chem. Int. Ed. 2010, 49, 7753–7756. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.H.; Kim, D.Y. Highly Enantioselective Conjugate Addition of 3-Substituted Oxindoles to Vinyl Sulfone Catalyzed by Binaphthyl-Modified Tertiary Amines. Synlett 2011, 1559–1562. [Google Scholar]
- Sarah, S.-M.; Alexakis, A. Chiral Amines as Organocatalysts for Asymmetric Conjugate Addition to Nitroolefins and Vinyl Sulfones via Enamine Activation. Chem. Commun. 2007, 3123–3135. [Google Scholar]
- Sarah, S.-M.; Alexakis, A.; Mareda, J.; Bollot, G.; Bernardinelli, G.; Filinchuk, Y. Enantioselective Organocatalytic Conjugate Addition of Aldehydes to Vinyl Sulfones and Vinyl Phosphonates as Challenging Michael Acceptors. Chem. Eur. J. 2009, 15, 3204–3220. [Google Scholar] [CrossRef]
- Alba, A.-N.R.; Company, X.; Valero, G.; Moyano, A.; Rios, R. Enantioselective Organocatalytic Addition of Oxazolones to 1,1-Bis(phenylsulfonyl)ethylene: A Convenient Asymmetric Synthesis of Quaternary α-Amino Acids. Chem. Eur. J. 2010, 16, 5354–5361. [Google Scholar]
- Bournaud, C.; Marchal, E.; Quintard, A.; Sarah, S.-M.; Alexakis, A. Organocatalyst-Mediated Enantioselective Intramolecular Michael Addition of Aldehydes to Vinyl Sulfones. Tetrahedron: Asymmetry 2010, 21, 1666–1673. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Tang, W.-H.; Chen, M.-X.; Wei, D.-K.; Dai, T.-L.; Shi, M. Highly Enantioselective Michael Addition of 3-Aryloxindoles to Phenyl Vinyl Sulfone Catalyzed by Cinchona Alkaloid-Derived Bifunctional Amine-Thiourea Catalysts Bearing Sulfonamide as Multiple Hydrogen-Bonding Donors. Eur. J. Org. Chem. 2011, 2011, 6078–6084. [Google Scholar]
- Bencivenni, G.; Wu, L.-Y.; Mazzanti, A.; Giannichi, B.; Pesciaioli, F.; Song, M.-P.; Bartoli, G.; Melchiorre, P. Targeting Structural and Stereochemical Complexity by Organocascade Catalysis: Construction of Spirocyclic Oxindoles Having Multiple Stereocenters. Angew. Chem. Int. Ed. 2009, 48, 7200–7203. [Google Scholar]
- Companyo, X.; Zea, A.; Alba, A.-N.R.; Mazzanti, A.; Moyano, A.; Rios, R. Organocatalytic Synthesis of Spiro Compounds via a Cascade Michael-Michael-Aldol Reaction. Chem. Commun. 2010, 46, 6953–6955. [Google Scholar] [CrossRef]
- Sun, W.; Hong, L.; Liu, C.; Wang, R. Asymmetric Construction of Quaternary Stereocenters by Direct Conjugate Addition of Oxindoles to Enone. Tetrahedron: Asymmetry 2010, 21, 2493–2497. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Enders, D. Asymmetric Michael Addition of N-Boc-Protected Oxindoles to Nitroalkenes Catalyzed by a Chiral Secondary Amine. Chem. Eur. J. 2012, 18, 4832–4835. [Google Scholar] [CrossRef]
- He, R.; Ding, C.; Maruoka, K. Phosphonium Salts as Chiral Phase-Transfer Catalysts: Asymmetric Michael and Mannich Reactions of 3-Aryloxindoles. Angew. Chem. Int. Ed. 2009, 48, 4559–4561. [Google Scholar] [CrossRef]
- Li, X.; Xi, Z.-G.; Luo, S.; Cheng, J.-P. Asymmetric Michael Reaction of 3-Substituted N-Boc Oxindoles to Activated Terminal Alkenes Catalyzed by a Bifunctional Tertiary-Amine Thiourea Catalyst. Org. Biomol. Chem. 2010, 8, 77–82. [Google Scholar] [CrossRef]
- Pesciaioli, F.; Tian, X.; Bencivenni, G.; Bartoli, G.; Melchiorre, P. Organocatalytic Asymmetric Conjugate Additions of Oxindoles and Benzofuranones to Cyclic Enones. Synlett 2010, 1704–1708. [Google Scholar]
- Freund, M.H.; Tsogoeva, S.B. l-Proline-Catalyzed Asymmetric Michael Addition of 2-Oxindoles to Enones: A Convenient Access to Oxindoles with a Quaternary Stereocenter. Synlett 2011, 503–507. [Google Scholar]
- Zheng, W.; Zhang, Z.; Kaplan, M.J.; Antilla, J.C. Chiral Calcium VAPOL Phosphate Mediated Asymmetric Chlorination and Michael Reactions of 3-Substituted Oxindoles. J. Am. Chem. Soc. 2011, 133, 3339–3341. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, M.H.; Kim, D.Y. Enantioselective Alkylation of β-Keto Esters by Phase-Transfer Catalysis using Chiral Quaternary Ammonium Salts. J. Org. Chem. 2004, 69, 6897–6899. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.Y. Enantio- and Diastereoselective Mannich-Type Reactions of α-Cyano Ketones with N-Boc Aldimines Catalyzed by Chiral Bifunctional Urea. Adv. Synth. Catal. 2009, 351, 1779–1782. [Google Scholar] [CrossRef]
- Kang, Y.K.; Kim, D.Y. Organocatalytic Highly Enantio- and Diastereoselective Mannich Reaction of β-Ketoesters with N-Boc-aldimines. J. Org. Chem. 2009, 74, 5734–5737. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.Y. Organocatalytic Highly Enantioselective Mannich Reaction of Fluoromaonate with N-Boc-aldimines. Synthesis 2010, 1860–1864. [Google Scholar]
- Kang, Y.K.; Kim, D.Y. Recent Advances in Catalytic Enantioselective Fluorination of Active Methines. Curr. Org. Chem. 2010, 14, 917–927. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kang, Y.K.; Kim, D.Y. Recent Advances in Catalytic Enantioselective Fluorination of Active Methines. Synlett 2011, 420–424. [Google Scholar]
- Kang, Y.K.; Kwon, B.K.; Mang, J.Y.; Kim, D.Y. Chiral Pd-catalyzed enantioselective Friedel–Crafts reaction of indoles with γ,δ-unsaturated β-keto phosphonates. Tetrahedron Lett. 2011, 52, 3247–3249. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Kang, Y.K.; Kim, D.Y. Microwave-Assisted Organocatalytic Synthesis of Tetrahydroquinolines via Hydride Transfer and Cyclization. Bull. Korean Chem. Soc. 2011, 32, 1773–1776. [Google Scholar] [CrossRef]
- Kang, Y.K.; Suh, K.H.; Kim, D.Y. Catalytic Enantioselective Friedel–Crafts Alkylation of Indoles with β,γ-Unsaturated α-Keto Phosphonates in the Presence of Chiral Palladium Complexes. Synlett 2011, 1125–1128. [Google Scholar]
- Kang, Y.K.; Yoon, S.J.; Kim, D.Y. Asymmetric Mannich-type Reactions of Fluorinated Ketoesters with Binaphthyl-Modified Thiourea Catalysts. Bull. Korean Chem. Soc. 2011, 32, 1195–1200. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.H.; Kim, D.Y. Organocatalytic Enantioselective α-Alkylation of Cyclic Ketones by SN1-Type Reaction of Alcohols. Bull. Korean Chem. Soc. 2011, 32, 1125–1126. [Google Scholar] [CrossRef]
- Kang, Y.K.; Kim, D.Y. Catalytic asymmetric Mannich-type reactions of fluorinated ketoesters with N-Boc aldimines in the presence of chiral palladium complexes. Tetrahedron Lett. 2011, 52, 2356–2358. [Google Scholar] [CrossRef]
- Moon, H.W.; Cho, M.J.; Kim, D.Y. Enantioselective Conjugate Addition of Fluorobis(phenylsulfonyl)methane to α,β-Unsaturated Ketones Catalyzed by Chiral Bifunctional Organocatalysts. Tetrahedron Lett. 2009, 50, 4896–4898. [Google Scholar] [CrossRef]
- Moon, H.W.; Kim, D.Y. Enantioselective Conjugate Addition of α-Nitroacetate to α,β-Unsaturated Ketones in Water. Tetrahedron Lett. 2010, 51, 2906–2908. [Google Scholar] [CrossRef]
- Kang, Y.K.; Kim, S.M.; Kim, D.Y. Enantioselective Organocatalytic C-H Bond Functionalization via Tandem 1,5-Hydride Transfer/Ring Closure: Asymmetric Synthesis of Tetrahydroquinolines. J. Am. Chem. Soc. 2010, 132, 11847–11849. [Google Scholar]
- Lee, J.H.; Bang, H.T.; Kim, D.Y. Catalytic Asymmetric α-Amination of α-Cyanoketones in the Presence of Chiral Palladium Complexes. Synlett 2008, 1821–1822. [Google Scholar]
- Mang, J.Y.; Kim, D.Y. Enantioselective α-Hydrazination of α-Fluoro-β-Ketoesters Using Bifunctional Organocatalysts. Bull. Korean Chem. Soc. 2008, 29, 2091–2092. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.H.; Kim, D.Y. Enantioselective Direct Amination of α-Cyanoketones Catalyzed by Bifunctional Organocatalysts. Synlett 2008, 2659–2662. [Google Scholar]
- Jung, S.H.; Kim, D.Y. Catalytic Electrophilic α-Hydrazination of β-Ketoesters Using Bifunctional Organocatalysts. Tetrahedron Lett. 2008, 49, 5527–5530. [Google Scholar] [CrossRef]
- Kim, D.Y. Catalytic Enantioselective Electrophilic α-Amination of α-Cyanoketones Catalyzed by Chiral Nickel Complexes. Bull. Korean Chem. Soc. 2008, 29, 2036–2038. [Google Scholar] [CrossRef]
- Mang, J.Y.; Kwon, D.G.; Kim, D.Y. Catalytic Asymmetric Electrophilic α-Amination of β-Ketoesters in the Presence of Chiral Nickel Complexes. Bull. Korean Chem. Soc. 2009, 30, 249–252. [Google Scholar] [CrossRef]
- Kwon, B.K.; Kim, S.M.; Kim, D.Y. Highly Enantioselective Conjugate Addition of Fluoromalonates to Nitroalkenes using Bifunctional Organocatalysts. J. Fluorine Chem. 2009, 130, 759–761. [Google Scholar] [CrossRef]
- Mang, J.Y.; Kwon, D.G.; Kim, D.Y. Enantioselective α-Hydrazination of α-Fluoro-β-Ketoesters Catalyzed by Chiral Nickel Complexes. J. Fluorine Chem. 2009, 130, 259–262. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, S.M.; Kim, D.Y. Organocatalytic Synthesis of Quaternary Stereocenter Bearing a Fluorine Atom: Enantioselective Conjugate Addition of α-Fluoro-β-ketoesters to Nitroalkenes. Tetrahedron Lett. 2009, 50, 4674–4676. [Google Scholar] [CrossRef]
- Kwon, B.K.; Kim, D.Y. Organocatalytic Asymmetric Michael Addition of β-Ketoesters to Nitroalkenes. Bull. Korean Chem. Soc. 2009, 30, 1441–1442. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, D.Y. Enantioselective Conjugate Addition of Fluoromalonate to Nitroalkenes Catalyzed by Chiral Nickel Complexes. Bull. Korean Chem. Soc. 2009, 30, 1439–1440. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, D.Y. Catalytic Enantioselective Fluorination of α-Chloro-β-ketoesters in the Presence of Chiral Nickel Complexes. Adv. Synth. Catal. 2010, 352, 2783–2786. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.M.; Kim, D.Y. Enantioselective Synthesis of Nitrocyclopropanes via Conjugate Addition of Bromomalonate to Nitroalkenes Catalyzed by Ni (II) Complexes. Tetrahedron Lett. 2012, 53, 3437–3439. [Google Scholar] [CrossRef]
- Lee, H.J.; Woo, S.B.; Kim, D.Y. Enantio- and Diastereoselective Michael Addition Reactions of α-Cyanoketones to Nitroalkenes Catalyzed by Binaphthyl-derived Organocatalyst. Tetrahedron Lett. 2012, 53, 3373–3377. [Google Scholar]
- Woo, S.B.; Kim, D.Y. Enantioselective Michael Addition of 2-Hydroxy-1,4-naphthoquinones to Nitroalkenes Catalyzed by Binaphthyl-derived Organocatalyst. Beilstein J. Org. Chem. 2012, 8, 699–704. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, H.J.; Woo, S.B.; Kim, D.Y. Enantioselective Michael Addition of 3-Aryl-Substituted Oxindoles to Methyl Vinyl Ketone Catalyzed by a Binaphthyl-Modified Bifunctional Organocatalyst. Molecules 2012, 17, 7523-7532. https://doi.org/10.3390/molecules17067523
Lee HJ, Woo SB, Kim DY. Enantioselective Michael Addition of 3-Aryl-Substituted Oxindoles to Methyl Vinyl Ketone Catalyzed by a Binaphthyl-Modified Bifunctional Organocatalyst. Molecules. 2012; 17(6):7523-7532. https://doi.org/10.3390/molecules17067523
Chicago/Turabian StyleLee, Hyun Joo, Saet Byeol Woo, and Dae Young Kim. 2012. "Enantioselective Michael Addition of 3-Aryl-Substituted Oxindoles to Methyl Vinyl Ketone Catalyzed by a Binaphthyl-Modified Bifunctional Organocatalyst" Molecules 17, no. 6: 7523-7532. https://doi.org/10.3390/molecules17067523