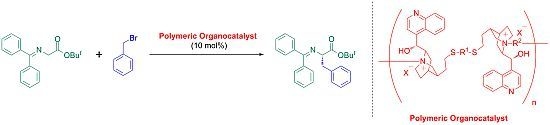
Synthesis of Main-Chain Chiral Quaternary Ammonium Polymers for Asymmetric Catalysis Using Quaternization Polymerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Thiolated Cinchonidine Quaternary Ammonium Dimers 5

2.2. Synthesis of Cinchonidine Dimers 7 Using Thiol-Ene Click Reaction

2.3. Synthesis of Main-Chain Chiral Quaternary Ammonium Polymers 8 Using Quaternization Polymerization

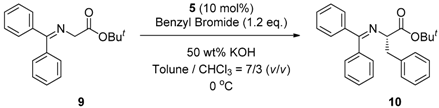
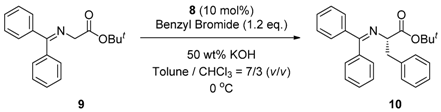
2.4. Asymmetric Benzylation of N-(Diphenylmethylidene)glycine tert-butyl Ester 9 Catalyzed by Main-Chain Chiral Quaternary Ammonium Polymers 8
| Entry | Catalyst | R | Time (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|
| 1 | 5a |  | 4 | 97 | 84 |
| 2 | 5b |  | 4 | 92 | 90 |
| 3 | 5c | 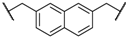 | 4 | 91 | 88 |
| 4 | 5d |  | 4 | 92 | 90
 |
| Entry | Catalyst | R1 | R2 | Time (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|---|
| 1 | 8a | 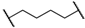 | 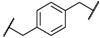 | 8 | 88 | 86 |
| 2 | 8b | 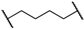 | 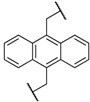 | 8 | 84 | 79 |
| 3 | 8c | 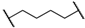 | 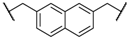 | 8 | 86 | 79 |
| 4 | 8d |  | 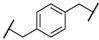 | 11 | 89 | 87 |
| 5 | 8e | 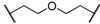 |  | 5 | 53 | 71 |
| 6 | 8f |  | 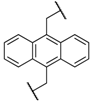 | 8 | 75 | 80 |
| 7 | 8g | 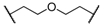 |  | 11 | 80 | 88 |
| 8 | 8h |  | 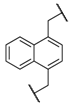 | 8 | 85 | 88
 |
3. Experimental
3.1. General
3.2. Preparation of Thiolated Chiral Quaternary Ammonium Salt Dimer 5
3.2.1. Preparation of Thiolated Cinchonidine 3
3.2.2. Preparation of Thiolated Chiral Quaternary Ammonium Salt Dimer 5
3.3. Preparation of Main-Chain Chiral Quaternary Ammonium Polymers 8
3.3.1. Preparation of Cinchonidine Dimer 7
3.3.2. Preparation of Main-Chain Chiral Quaternary Ammonium Polymers 8
3.4. General Procedure for Asymmetric Benzylation of N-(Diphenylmethylidene)glycine tert-butyl Ester 9 Catalyzed by Main-Chain Chiral Quaternary Ammonium Polymers 8
4. Conclusions
Acknowledgments
References and Notes
- Cinchona Alkaloids in Synthesis & Catalysis; Song, C.E. (Ed.) Wiley-VCH: Weinheim, Germany, 2009.
- Elizabeth, M.O.; Yeboah, S.O.; Girija, S.S. Recent applications of Cinchona alkaloids and their derivatives as catalysts in metal-free asymmetric synthesis. Tetrahedron Lett. 2011, 67, 1725–1762. [Google Scholar]
- Marcelli, T.; Hiemstra, H. Cinchona alkaloids in asymmetric organocatalysis. Synthesis 2010, 8, 1229–1279. [Google Scholar] [CrossRef]
- Ooi, T.; Maruoka, K. Recent advances in asymmetric phase-transfer catalysis. Angew. Chem. Int. Ed. Engl. 2007, 46, 4222–4266. [Google Scholar] [CrossRef]
- Dolling, U.-H.; Davis, P.; Grabowski, E.J.J. Efficient catalytic asymmetric alkylations. 1. Enantioselective synthesis of (+)-indacrinone via chiral phase-transfer catalysis. J. Am. Chem. Soc. 1984, 106, 446–447. [Google Scholar]
- O’Donnell, M.J.; Bennett, W.D.; Wu, S. The stereoselective synthesis of α-amino acids by phase-transfer catalysis. J. Am. Chem. Soc. 1989, 111, 2353–2355. [Google Scholar] [CrossRef]
- Corey, E.J.; Zhang, F.-Y. re- and si-Face-Selective nitroaldol reactions catalyzed by a rigid chiral quaternary ammonium salt: A highly stereoselective synthesis of the HIV protease inhibitor amprenavir (Vertex 478). Angew. Chem. Int. Ed. Engl. 1999, 38, 1931–1934. [Google Scholar] [CrossRef]
- Horikawa, M.; Busch-Peterson, J.; Corey, E.J. Enantioselective synthesis of β-hydroxy-α-amino acid esters by aldol coupling using a chiral quaternary ammonium salt as catalyst. Tetrahedron Lett. 1999, 40, 3843–3846. [Google Scholar] [CrossRef]
- Sasai, H.; Itoh, N.; Suzuki, T.; Shibasaki, M. Catalytic asymmetric nitroaldol reaction: An efficient synthesis of (S) propranolol using the lanthanum binaphthol complex. Tetrahedron Lett. 1993, 34, 855–858. [Google Scholar] [CrossRef]
- Fini, F.; Bernardi, L.; Herrera, R.P.; Petterson, D.; Ricci, A.; Sgarzani, V. Phase-transfer-catalyzed enantioselective mannich reaction of malonates with α-Amido sulfones. Adv. Synth. Catal. 2006, 348, 2043–2046. [Google Scholar] [CrossRef]
- Fini, F.; Sgarzani, V.; Petterson, D.; Herrera, R.P.; Bernardi, L.; Ricci, A. Phase-transfer-catalyzed asymmetric Aza-henry reaction using N-carbamoyl imines generated in situ from α-Amido sulfones. Angew. Chem. Int. Ed. Engl. 2005, 44, 7975–7978. [Google Scholar] [CrossRef]
- Hummelen, J.C.; Wynberg, H. Alkaloid assisted asymmetric synthesis IV additional routes to chiral epoxides. Tetrahedron Lett. 1978, 19, 1089–1092. [Google Scholar] [CrossRef]
- Herrera, R.P.; Sgarzani, V.; Bernardi, L.; Fini, F.; Petterson, D.; Ricci, A. Phase transfer catalyzed enantioselective strecker reactions of α-Amido sulfones with cyanohydrins. J. Org. Chem. 2006, 71, 9869–9872. [Google Scholar] [CrossRef]
- Masui, M.; Ando, A.; Shioiri, T. New methods and reagents in organic synthesis. 75. Asymmetric synthesis of α-hydroxy ketones using chiral phase transfer catalysts. Tetrahedron Lett. 1988, 29, 2835–2838. [Google Scholar]
- Shibata, N.; Suzuki, E.; Takeuchi, Y. A fundamentally new approach to enantioselective fluorination based on cinchona alkaloid derivatives/selectfluor combination. J. Am. Chem. Soc. 2000, 122, 10728–10729. [Google Scholar] [CrossRef]
- Conn, R.S.E.; Lovell, A.V.; Karady, S.; Weinstock, L.M. Chiral Michael addition: Methyl vinyl ketone addition catalyzed by Cinchona alkaloid derivatives. J. Org. Chem. 1986, 51, 4710–4711. [Google Scholar] [CrossRef]
- Corey, E.J.; Noe, M.C.; Xu, F. Highly enantioselective synthesis of cyclic and functionalized α-amino acids by means of a chiral phase transfer catalyst. Tetrahedron Lett. 1998, 39, 5347–5350. [Google Scholar] [CrossRef]
- Helder, R.; Hummelen, J.C.; Lanne, R.W.P.M.; Wiering, J.S.; Wynberg, H. Catalytic asymmetric induction in oxidation reactions. The synthesis of optically active epoxides. Tetrahedron Lett. 1976, 17, 1831–1834. [Google Scholar] [CrossRef]
- Arai, S.; Tsuge, H.; Shioiri, T. Asymmetric epoxidation of α,β-unsaturated ketones under phase-transfer catalyzed conditions. Tetrahedron Lett. 1998, 39, 7563–7566. [Google Scholar] [CrossRef]
- Aires-de-Sousa, J.; Lobo, A.M.; Prabhakar, S. A new enantioselective synthesis of N-arylaziridines by phase-transfer catalysis. Tetrahedron Lett. 1996, 37, 3183–3186. [Google Scholar] [CrossRef]
- Murugan, E.; Siva, A. Synthesis of asymmetric N-Arylaziridine derivatives using a new chiral phase-transfer catalyst. Synthesis 2005, 2022–2028. [Google Scholar] [CrossRef]
- Balcells, J.; Colonna, S.; Fornasier, R. Asymmetric induction in the borohydride reduction of carbonyl compounds by means of a chiral phase-transfer catalyst. Synthesis 1976, 1976, 266–267. [Google Scholar] [CrossRef]
- Drew, M.D.; Lawrence, N.J.; Watson, W.; Bowles, S.A. The asymmetric reduction of ketones using chiral ammonium fluoride salts and silanes. Tetrahedron Lett. 1997, 38, 5857–5860. [Google Scholar]
- Haraguchi, N.; Itsuno, S. Polymer-Immobilized Chiral Organocatalyst. In Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis; Itsuno, S., Ed.; Wiley: Singapore, 2011; pp. 17–61. [Google Scholar]
- Altava, B.; Burguete, M.I.; Luis, S.V. Polymer-Supported Organocatalysts. In The Power of Functional Resins in Organic, Synthesis; Puche, J.T., Albericio, F., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 247–308. [Google Scholar]
- Cozzi, F. Immobilization of organic catalysts: When, why, and how. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-supported organic catalysts. Chem. Rev. 2003, 103, 3401–3430. [Google Scholar] [CrossRef]
- Zhengpu, Z.; Yongmer, W.; Zhen, W.; Hodge, P. Asymmetric synthesis of α-amino acids using polymer-supported chiral phase transfer catalysts. React. Funct. Polym. 1999, 41, 37–43. [Google Scholar] [CrossRef]
- Chinchilla, R.; Mazón, P.; Nájera, C. Asymmetric synthesis of α-amino acids using polymer-supported Cinchona alkaloid-derived ammonium salts as chiral phase-transfer catalysts. Tetrahedron: Asymmetry 2000, 11, 3277–3281. [Google Scholar]
- Thierry, B.; Plaquevent, J.-C.; Cahard, D. New polymer-supported chiral phase-transfer catalysts in the asymmetric synthesis of α-amino acids: The role of a spacer. Tetrahedron: Asymmertry 2001, 12, 983–986. [Google Scholar]
- Thierry, B.; Perrard, T.; Audourd, C.; Plaquevent, J.-C.; Cahard, D. Solution- and solid-phase approaches in asymmetric phase-transfer catalysis by cinchona alkaloid derivatives. Synthesis 2001, 2001, 1742–1746. [Google Scholar] [CrossRef]
- Danelli, T.; Annunziata, R.; Benaglia, M.; Cinquini, M.; Cozzi, F.; Tocco, G. Immobilization of catalysts derived from Cinchona alkaloids on modified poly(ethylene glycol). Tetrahedron: Asymmetry 2003, 14, 461–467. [Google Scholar]
- Thierry, B.; Plaquevent, J.-C.; Cahard, D. Poly(ethylene glycol) supported cinchona alkaloids as phase transfer catalysts: Application to the enantioselective synthesis of α-amino acids. Tetrahedron: Asymmetry 2003, 14, 1671–1677. [Google Scholar]
- Chinchilla, R.; Mazón, P.; Nájera, C. Polystyrene-anchored Cinchona ammonium salts: Easily recoverable phase-transfer catalysts for the asymmetric synthesis of α-amino acids. Adv. Synth. Catal. 2004, 346, 1186–1194. [Google Scholar] [CrossRef]
- Lv, J.; Wang, X.; Liu, J.; Zhang, L.; Wang, Y. Catalytic asymmetric epoxidation of chalcones under poly(ethylene glycol)-supported Cinchona ammonium salt catalyzed conditions. Tetrahedron: Asymmetry 2006, 17, 330–335. [Google Scholar]
- Wang, X.; Yin, L.; Wang, Y. Synthesis of new dimeric-PEG-supported cinchona ammonium salts as chiral phase transfer catalysts for the alkylation of Schiff bases with water as the solvent. Tetrahedron Asymmetry 2007, 18, 108–114. [Google Scholar] [CrossRef]
- Arakawa, Y.; Haraguchi, N.; Itsuno, S. An Immobilization Method of Chiral Quaternary Ammonium Salts onto Polymer Supports. Angew. Chem. Int. Ed. Engl. 2008, 47, 8232–8235. [Google Scholar] [CrossRef]
- Shi, Q.; Lee, Y.-J.; Song, H.; Cheng, M.; Jew, S.-S.; Park, H.-G.; Jeong, B.-S. Electronically modified polymer-supported cinchona phase-transfer catalysts for asymmetric synthesis of α-alkyl-α-amino acid derivatives. Chem. Lett. 2008, 37, 436–437. [Google Scholar] [CrossRef]
- Haraguchi, N.; Takemura, Y.; Itsuno, S. Novel polymer-supported organocatalyst via ion exchange reaction: Facile immobilization of chiral imidazolidin-4-one and its application to Diels-Alder reaction. Tetrahedron Lett. 2010, 51, 1205–1208. [Google Scholar] [CrossRef]
- Itsuno, S.; Paul, D.K.; Ishimoto, M.; Haraguchi, N. Designing chiral quaternary ammonium polymers: Novel type of polymeric catalyst for asymmetric alkylation reaction. Chem. Lett. 2010, 39, 86–87. [Google Scholar] [CrossRef]
- Itsuno, S.; Paul, D.K.; Haraguchi, N. Main-chain ionic polymers: Synthesis of optically active quaternary ammonium sulfonate polymers and their application in asymmetric catalysis. J. Am. Chem. Soc. 2010, 132, 2864–2865. [Google Scholar] [CrossRef]
- Itsuno, S.; Parvez, M.M.; Haraguchi, N. Polymeric chiral organocatalysts. Polym. Chem. 2011, 2, 1942–1949. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haraguchi, N.; Itsuno, S. Molecular design of chiral quaternary ammonium polymers for asymmetric catalysis applications. Org. Biomol. Chem. 2012, 10, 2870–2877. [Google Scholar] [CrossRef]
- Parvez, M.M.; Salam, M.A.; Haraguchi, N.; Itsuno, S. Synthesis of chiral ionic polymers containing quaternary ammonium sulfonate structure and their catalytic activity in asymmetric alkylation. J. Chin. Chem. Soc. 2012. [Google Scholar] [CrossRef]
- Haraguchi, N.; Kiyono, H.; Takemura, Y.; Itsuno, S. Design of main-chain polymer of chiral imidazolidinone for asymmetric organocatalysis application. Chem. Commun. 2012, 48, 4011–4013. [Google Scholar] [CrossRef]
- Hodge, P.; Khoshdel, E.; Waterhouse, J.; Fréchet, J.M.J. Michael additions catalysed by cinchona alkaloids bound via their vinyl groups to preformed crosslinked polymers. J. Chem. Soc. Perkin Trans. 1 1985, 2327–2331. [Google Scholar]
- Griesbaum, K. Probleme und Möglichkeiten der radikalischen Addition von Thiolen an ungesättigte Verbindungen. Angew. Chem. 1970, 82, 276–290. [Google Scholar] [CrossRef]
- Griesbaum, K. Problems and possibilities of the free-radical addition of thiols to unsaturated compounds. Angew. Chem. Int. Ed. Engl. 1970, 9, 273–287. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol-enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds of are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Haraguchi, N.; Ahamed, P.; Parvez, M.M.; Itsuno, S. Synthesis of Main-Chain Chiral Quaternary Ammonium Polymers for Asymmetric Catalysis Using Quaternization Polymerization. Molecules 2012, 17, 7569-7583. https://doi.org/10.3390/molecules17067569
Haraguchi N, Ahamed P, Parvez MM, Itsuno S. Synthesis of Main-Chain Chiral Quaternary Ammonium Polymers for Asymmetric Catalysis Using Quaternization Polymerization. Molecules. 2012; 17(6):7569-7583. https://doi.org/10.3390/molecules17067569
Chicago/Turabian StyleHaraguchi, Naoki, Parbhej Ahamed, Md. Masud Parvez, and Shinichi Itsuno. 2012. "Synthesis of Main-Chain Chiral Quaternary Ammonium Polymers for Asymmetric Catalysis Using Quaternization Polymerization" Molecules 17, no. 6: 7569-7583. https://doi.org/10.3390/molecules17067569