A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry



2.2. In Vitro Cytotoxicity Assay

3. Experimental
3.1. Chemistry
3.1.1. General
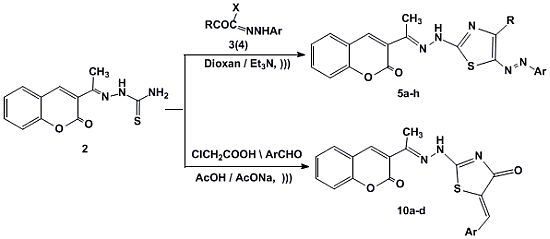
3.1.2. Synthesis of 3-[1-(4-Substituted-5-(aryldiazenyl)thiazol-2-yl)hydrazono) ethyl]-2H-chromen-2-ones 5a–h
3.1.2.1. Method A: General Procedure
3.1.2.2. Method B
3.1.2.2.1. Synthesis of 3-[1-((4-Substituted thiazol-2-yl)hydrazono)ethyl]-2H-chromen-2-ones 8,9
3.1.2.2.2. Coupling of 8(9) with Arenediazonium Chlorides
3.1.3. Synthesis of 5-Arylidene-2-(2-(1-(2-oxo-2H-chromen-3-yl)ethylidene)hydrazinyl)thiazol-4(5H)-ones 10a–d
3.1.3.1. Method A
3.1.3.2. Method B
3.1.3.2.1. Synthesis of 2-[2-(1-(2-oxo-2H-chromen-3-yl)ethylidene)hydrazinyl]thiazol-4(5H)-one (11)
3.1.3.2.2. Reaction of 8 with Aromatic Aldehydes
3.1.4. Synthesis of 2-Cyano-N'-(1-(2-oxo-2H-chromen-3-yl)ethylidene)acetohydrazide (12)
3.1.5. Reaction of 11 with Aromatic Aldehydes
3.2. Cytotoxic Activity
4. Conclusions
Acknowledgements
References
- Czerpack, R.; Skolska, S. Effect of selected synthetic regulators on Pseudomonas aeruginosa growth in liquid culture. Med. Dosw. Microbiol. 1982, 34, 37–50. [Google Scholar]
- Jund, L.; Corse, J.; King, A.S.; Bayne, H.; Mihrag, K. Antimicrobial properties of 6,7-dihydroxy-7,8-dihydroxy-, 6-hydroxy- and 8-hydroxycoumarins. Phytochemistry 1971, 10, 2971–2974. [Google Scholar] [CrossRef]
- El-Ansary, S.L.; Aly, E.I.; Halem, M.A. New coumarin derivatives as antibacterial agents. Egypt J. Pharm. Sci. 1992, 33, 379–390. [Google Scholar]
- Reddy, Y.D.; Somayojulu, V.V. Synthesis, spectra and physiological activity of 7H-pyrano[3,2-c]benzoxazole-7-one. J. Ind. Chem. Soc. 1981, 58, 599–601. [Google Scholar]
- Weber, U.S.; Steffen, B.; Siegers, C. Antitumor activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol. Pharmacol. 1998, 99, 93–206. [Google Scholar]
- Küçükgüzel, G.; Kocatepe, A.; Clercq, E.D.; Şahin, F.; Güllüce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353–359. [Google Scholar]
- Verma, A.; Saraf, S.K. 4-Thiazolidinone-Abiologicallyactivescaffold. Eur. J. Med. Chem. 2008, 43, 897–905. [Google Scholar] [CrossRef]
- Akerblom, E.B. Synthesis and structure-activity relations of a series of antibacterially active 5-(5-nitro-2-furfurylidene)thiazolones, 5-(5-nitro-2-furylpropenylidene)thiazolones, and 6-(5-nitro-2-furyl)-4H-1,3-thiazinones. J. Med. Chem. 1974, 17, 609–615. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, S.F.; Amr, A.El-G.E.; Abdalla, M.M. Synthesis and reactions of thiosemicarbazides, Triazoles, and Schiff bases as antihypertensive α-blocking agents. Monatsh. Chem. 2008, 139, 1083–1090. [Google Scholar]
- Amir, M.; Agarwal, R. Synthesis and antibacterial activity of 1-thiocarbamoyl-3-methyl-4- arylhydrazono)-2-pyrazolin-5-one. J. Ind. Chem. Soc. 1997, 74, 154–155. [Google Scholar]
- Gholap, A.R.; Venkatesan, K.; Daniel, T.R.; Lahoti, J.; Srinivasan, K.V. Ionic liquid promoted novel and efficient one pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones at ambient temperature under ultrasound irradiation. Green Chem. 2004, 6, 147. [Google Scholar] [CrossRef]
- Bazgir, A.; Ahadi, S.; Ghahremanzadeh, R.; Khavasi, H.R.; Mirzaei, P. Ultrasound-assisted one-pot, three-component synthesis of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine]-2,6′(1′H)-diones in water. Ultrason. Sonochem. 2010, 17, 447–452. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, J.J.; Li, T.S. Ultrasound-assisted synthesis of pyrroles catalyzed by zirconium chloride under solvent-free conditions. Ultrason. Sonochem. 2008, 15, 673–676. [Google Scholar] [CrossRef]
- Abd-el-Rahman, N.M.; Saleh, T.S.; Mady, M.F. Ultrasound assisted synthesis of some new 1,3,4-thiadiazole and bi(1,3,4-thiadiazole) derivatives incorporating pyrazolone moiety. Ultrason. Sonochem. 2009, 16, 70–74. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.; Qu, L.; Wang, J.; Yuan, J. Chen, S.; Li, X.; Qu, C. Ultrasonic-assisted synthesis of chrysin derivatives linked with 1,2,3-triazoles by 1,3-dipolar cycloaddition reaction. Ultrason. Sonochem. 2011, 18, 527–533. [Google Scholar] [CrossRef]
- Mahdavinia, G.H.; Rostamizadeh, S.; Amani, A.M.; Emdadi, Z. Ultrasound-promoted greener synthesis of aryl-14-H-dibenzo[a,j]xanthenes catalyzed by NH4H2PO4/SiO2 in water. Ultrason. Sonochem. 2009, 16, 7–10. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new heterocycles derived from 3-(3-methyl-1H-indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull. Korean Chem. Soc. 2012, in press. [Google Scholar]
- Riyadh, S.M.; Farghaly, T.A.; Gomha, S.M. Novel polyazaheterocyclic systems: Synthesis, antitumor, and antimicrobial Activities. Arch. Pharm. Res. 2010, 33, 1721–1728. [Google Scholar] [CrossRef]
- Gomha, S.M.; Hassaneen, H.M.E. Synthesis and antimicrobial activity of some new pyrazoles, fused pyrazolo[3,4-d]-pyrimidine and 1, 2-dihydroimidazo-[2,1-c][1,2,4]triazin-6-one derivatives. Molecules 2011, 16, 6549–6560. [Google Scholar] [CrossRef]
- Chimenti, F.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Investigations on the 2-thiazolylhydrazyne scaffold: Synthesis and molecular modeling of selective human monoamine oxidase inhibitors. Bioorg. Med. Chem. 2010, 18, 5715–5723. [Google Scholar]
- Hantzsch, A.; Weber, J.H. Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe). Dtsch. Chem. Ges. 1887, 20, 3118–3132. [Google Scholar] [CrossRef]
- Metwally, M.A.; Abdel-latif, E.; Amer, F.A.; Kaupp, G. Synthesis of new 5-thiazolyl azo-disperse dyes for dyeing polyester fabrics. Dyes Pigments 2004, 60, 249–264. [Google Scholar] [CrossRef]
- Metwally, M.A.; Abdel-Latif, E.; Amer, F.A. New 4-arylazo-2-(substituted)-3-phenyl-1,3- thiazolidin-5-ones as disperse dyes part 1. J. Text. Assoc. 2001, 63, 155–159. [Google Scholar]
- Zhou, J.F.; Gong, G.X.; Zhu, F.X.; Zhi, S. Microwave promoted one-pot synthesis of 3-(2′-amino-3′-cyano-4′-arylpyrid-6′-yl) coumarins. J. Chin. Chem. Lett. 2009, 20, 37–39. [Google Scholar] [CrossRef]
- Volmajer, J.; Toplak, R.; Leban, I.; LeMarechal, A.M. Synthesis of new iminocoumarins and their transformations into N-chloro and hydrazono compounds. Tetrahedron 2005, 61, 7012–7021. [Google Scholar]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles. Part II. New routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdelhamid, A.O. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–327. [Google Scholar]
- Sample Availability: Samples of the synthesized compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gomha, S.M.; Khalil, K.D. A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity. Molecules 2012, 17, 9335-9347. https://doi.org/10.3390/molecules17089335
Gomha SM, Khalil KD. A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity. Molecules. 2012; 17(8):9335-9347. https://doi.org/10.3390/molecules17089335
Chicago/Turabian StyleGomha, Sobhi M., and Khaled D. Khalil. 2012. "A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity" Molecules 17, no. 8: 9335-9347. https://doi.org/10.3390/molecules17089335