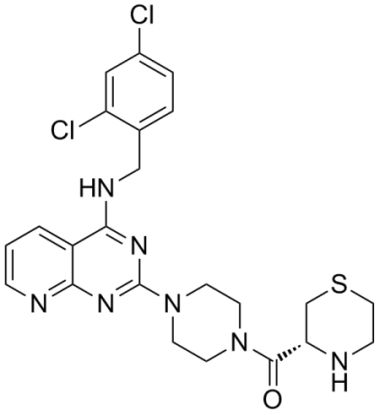
Design and Synthesis of a Series of Pyrido[2,3-d]pyrimidine Derivatives as CCR4 Antagonists
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Chemotaxis Inhibition Assay


| Compound | BMS-397 | 6b | 7a | 7b | 7c | 7d | 8 |
|---|---|---|---|---|---|---|---|
| CCR4-MDC | 60.3 ± 3.5 | 61.4 ± 1.0 | 52.2 ± 4.3 | 40.0 ± 4.9 | 32.0 ± 0.3 | 29.7 ± 3.7 | 23.3 ± 6.7 |
| CCR4-TARC | 59.2 ± 4.0 | 57.3 ± 4.7 | 48.8 ± 5.4 | 47.7 ± 7.2 | 48.7 ± 6.3 | 48.1 ± 6.6 | 34.8 ± 0.6 |
| CCR4-C27 | 9.6 ± 3.5 | 21.4 ± 1.5 | 16.8 ± 5.2 | 35.7 ± 7.0 | 34.7 ± 0.7 | 37.1 ± 1.8 | −1.5 ± 8.8 |
| CCR3-CCL11 | 2.9 ± 8.9 | ||||||
| CCR5-CCL5 | −7.4 ± 41.4 | ||||||
| CXCR1-IL-8 | 2.9 ± 5.1 | ||||||
| CXCR4-SDF-1 | −4.8 ± 3.2 |
2.3. Effects of 6b Administration on Symptoms of Murine Allergic Rhinitis
2.4. Determination of Acute Toxicity
3. Experimental
3.1. Chemistry
3.1.1. Materials and Reagents
3.1.2. Chemical Synthesis
3.2. Bioassay Methods for Chemotaxis Inhibition
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 6b, 7a–d, and 8 are available from the authors.
References
- Vijayanand, P.; Durkin, K.; Hartmann, G.; Morjaria, J.; Seumois, G.; Staples, K.J.; Hall, D.; Bessant, C.; Bartholomew, M.; Howarth, P.H.; et al. Chemokine receptor 4 plays a key role in T cell recruitment into the airways of asthmatic patients. J. Immunol. 2010, 184, 4568–4574. [Google Scholar]
- Imai, T.; Yoshida, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Yoshie, O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J. Biol. Chem. 1996, 271, 21514–21521. [Google Scholar]
- Godiska, R.; Chantry, D.; Raport, C.J.; Sozzani, S.; Allavena, P.; Leviten, D.; Mantovani, A.; Gray, P.W. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1997, 185, 1595–1604. [Google Scholar] [CrossRef]
- Li, F.; Song, Q.; Di, C.; Wang, L.; Shi, Q.; Sun, R.; Xia, D.; Rui, M.; Tang, J.; Ma, D. Molecular cloning and characterization of chemokine-like factor 1 (CKLF1), a novel human cytokine with unique structure and potential chemotactic activity. Biochem. J. 2001, 357, 127–135. [Google Scholar] [CrossRef]
- Perros, F.; Hoogsteden, H.C.; Coyle, A.J.; Lambrecht, B.N.; Hammad, H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy 2009, 64, 995–1002. [Google Scholar] [CrossRef]
- Kawasaki, S.; Takizawa, H.; Yoneyama, H.; Nakayama, T.; Fujisawa, R.; Izumizaki, M.; Imai, T.; Yoshie, O.; Homma, I.; Kazuhiko, Y.; et al. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 2001, 166, 2055–2062. [Google Scholar]
- Gonzalo, J.-A.; Pan, Y.; Lloyd, C.M.; Jia, G.-Q.; Yu, G.; Dussault, B.; Powers, C.A.; Proudfoot, A.E.I.; Coyle, A.J.; Gearing, D.; et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 1999, 163, 403–411. [Google Scholar]
- Stolberg, V.R.; Chiu, B.C.; Schmidt, B.M.; Kunkel, S.L.; Sandor, M.; Chensue, S.W. CC chemokine receptor 4 contributes to innate NK and chronic stage T helper cell recall responses during Mycobacterium bovis infection. Am. J. Pathol. 2011, 178, 233–244. [Google Scholar] [CrossRef]
- Tan, Y.; Han, W.; Chen, Y.; Ouyang, N.; Tang, Y.; Li, F.; Ding, P.; Ren, X.; Zeng, G.; Ding, J.; et al. Chemokine-like factor 1, a novel cytokine, contributes to airway damage, remodeling and pulmonary fibrosis. Chin. Med. J. 2004, 117, 1123–1129. [Google Scholar]
- Han, W.; Luo, Y.; Tang, J.; Zhang, Y.; Chen, Y.; Li, Y.; Gu, W.; Huang, J.; Gui, L.; Tang, Y.; et al. Two C-terminal peptides of human CKLF1 interact with the chemokine receptor CCR4. Int. J. Biochem. Cell Biol. 2008, 40, 909–919. [Google Scholar] [CrossRef]
- Purandare, A.V.; Wan, H.; Somerville, J.E.; Burke, C.; Vaccaro, W.; Yang, X.; Mcintyre, K.W.; Poss, M.A. Core exploration in optimization of chemokine receptor CCR4 antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 679–682. [Google Scholar]
- Purandare, A.V.; Wan, H.; Gao, A.; Somerville, J.; Burke, C.; Vaccaro, W.; Yang, X.; Mcintyre, K.W.; Poss, M.A. Optimization of CCR4 antagonists: Side-chain exploration. Bioorg. Med. Chem. Lett. 2006, 16, 204–207. [Google Scholar]
- Purandare, A.V.; Gao, A.; Wan, H.; Somerville, J.; Burke, C.; Seachord, C.; Vaccaro, W.; Wityak, J.; Poss, M.A. Identification of chemokine receptor CCR4 antagonist. Bioorg. Med. Chem. Lett. 2005, 15, 2669–2672. [Google Scholar] [CrossRef]
- Purandare, A.V.; Somerville, J.E. Antagonists of CCR4 as immunomodulatory agents. Curr. Top. Med. Chem. 2006, 6, 1335–1344. [Google Scholar]
- Purandare, A.V. Preparation of Piperazines as Chemokine Receptor Antagonists. WO Patent WO/2004/020584, 11 March 2004. [Google Scholar]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hamaguchi, W.; Yamasaki, S.; Koganemaru, Y.; Hattori, K.; Miyazaki, T.; et al. Potent and orally bioavailable CCR4 antagonists: Synthesis and structure-activity relationship study of 2-aminoquinazolines. Bioorg. Med. Chem. 2009, 17, 64–73. [Google Scholar] [CrossRef]
- Burdi, D.F.; Chi, S.; Mattia, K.; Harrington, C.; Shi, Z.; Chen, S.; Jacutin-Porte, J.; Bennett, R.; Carson, K.; Yin, W.; et al. Small molecule antagonists of the CC chemokine receptor 4 (CCR4). Bioorg. Med. Chem. Lett. 2007, 17, 3141–3145. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Xu, Q.; Mahmud, H.; Houze, J.; Zhu, L.; Akerman, M.; Tonn, G.; Tang, L.; Mcmaster, B.E.; et al. Optimization of 2-aminothiazole derivatives as CCR4 antagonists. Bioorg. Med. Chem. Lett. 2006, 16, 2800–2803. [Google Scholar] [CrossRef]
- Newhouse, B.; Allen, S.; Fauber, B.; Anderson, A.S.; Eary, C.T.; Hansen, J.D.; Schiro, J.; Gaudino, J.J.; Laird, E.; Chantry, D.; et al. Racemic and chiral lactams as potent, selective and functionally active CCR4 antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 5537–5542. [Google Scholar] [CrossRef]
- Kuhn, C.F.; Bazin, M.; Philippe, L.; Zhang, J.; Tylaska, L.; Miret, J.; Bauer, P.H. Bipiperidinyl carboxylic acid amides as potent, selective, and functionally active CCR4 antagonists. Chem. Biol. Drug Des. 2007, 70, 268–272. [Google Scholar] [CrossRef]
- Sanmartin, C.; Echeverria, M.; Mendivil, B.; Cordeu, L.; Cubedo, E.; Foncillas, J.; Font, M.; Palop, J.A. Synthesis and biological evaluation of new symmetrical derivatives as cytotoxic agents and apoptosis inducers. Bioorg. Med. Chem. 2005, 13, 2031–2044. [Google Scholar] [CrossRef]
- Li, G.; Wang, D.; Sun, M.; Li, G.; Hu, J.; Zhang, Y.; Yuan, Y.; Ji, H.; Chen, N.; Liu, G. Discovery and optimization of novel 3-piperazinylcoumarin antagonist of chemokine-like factor 1 with oral antiasthma activity in mice. J. Med. Chem. 2010, 53, 1741–1754. [Google Scholar] [CrossRef]
- Qi, H.; Zheng, Y.; Xu, E.; Guo, C.; Zhang, Y.; Sun, Q.; Xiao, J.; Ma, D.; Wang, Y. An antagonist for CCR4 alleviates murine allergic rhinitis by intranasal administration. Int. Arch. Allergy Immunol. 2012, 159, 297–305. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gong, H.; Qi, H.; Sun, W.; Zhang, Y.; Jiang, D.; Xiao, J.; Yang, X.; Wang, Y.; Li, S. Design and Synthesis of a Series of Pyrido[2,3-d]pyrimidine Derivatives as CCR4 Antagonists. Molecules 2012, 17, 9961-9970. https://doi.org/10.3390/molecules17089961
Gong H, Qi H, Sun W, Zhang Y, Jiang D, Xiao J, Yang X, Wang Y, Li S. Design and Synthesis of a Series of Pyrido[2,3-d]pyrimidine Derivatives as CCR4 Antagonists. Molecules. 2012; 17(8):9961-9970. https://doi.org/10.3390/molecules17089961
Chicago/Turabian StyleGong, Hongwei, Hui Qi, Wei Sun, Yang Zhang, Dan Jiang, Junhai Xiao, Xiaohong Yang, Ying Wang, and Song Li. 2012. "Design and Synthesis of a Series of Pyrido[2,3-d]pyrimidine Derivatives as CCR4 Antagonists" Molecules 17, no. 8: 9961-9970. https://doi.org/10.3390/molecules17089961