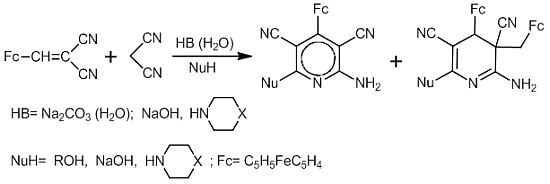
4-Ferrocenylpyridine- and 4-Ferrocenyl-3-ferrocenylmethyl-3,4-dihydropyridine-3,5-dicarbonitriles: Multi-Component Synthesis, Structures and Electrochemistry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 6-Alkoxy-2-amino-4-ferrocenylpyridine-3,5-dicarbonitriles and 6-alkoxy-2-amino-4-ferrocenyl-3-ferrocenylmethyl-3,4-dihydropyridine-3,5-dicarbonitriles

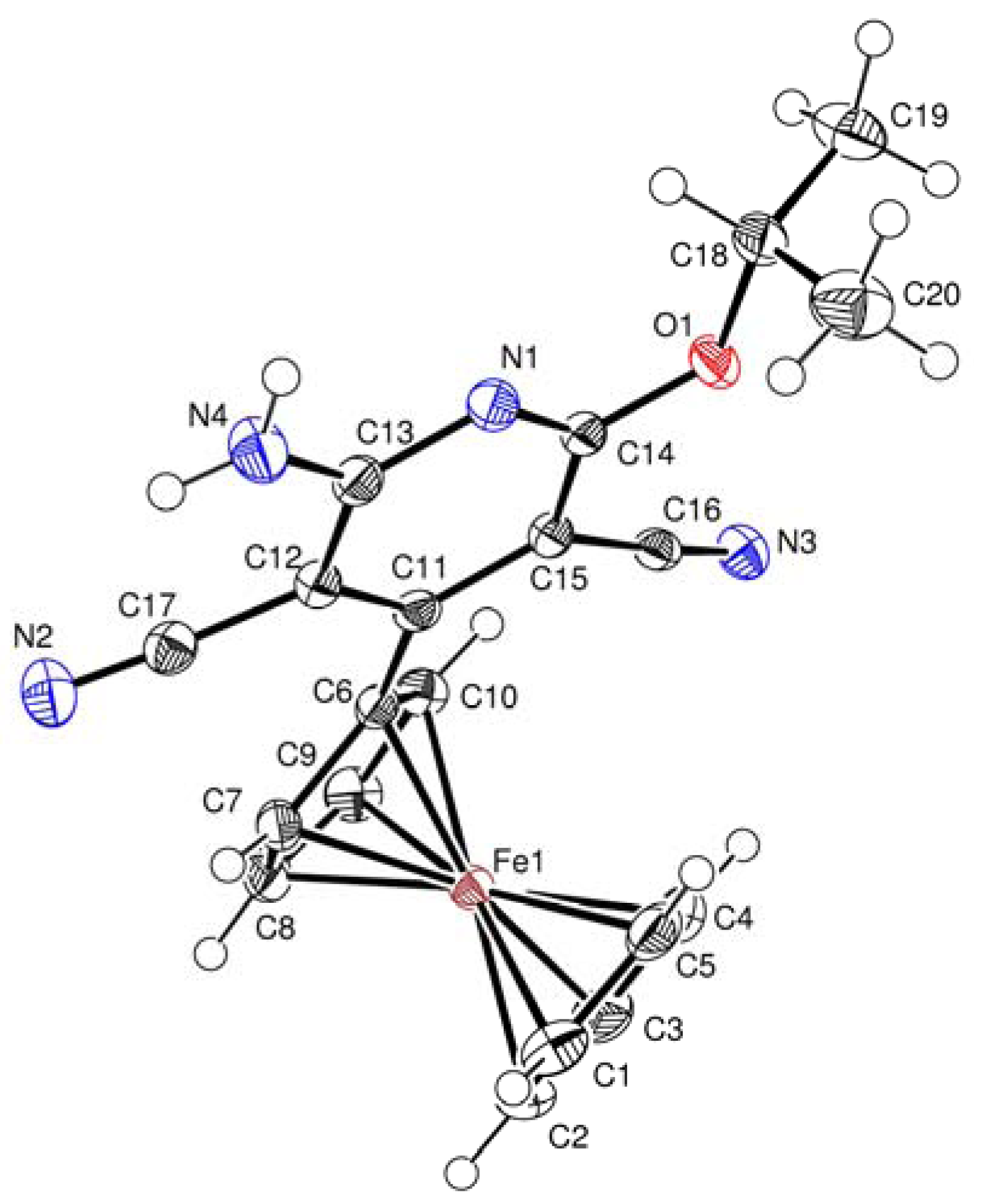


| Selected bond lengths (Å) | Selected bond angles (°) | |||||
|---|---|---|---|---|---|---|
| 3b | ||||||
| N(1)-C(13) | 1.346(2) | C(15)-C(11)-C(12) | 116.21(15) | |||
| N(1)-C(14) | 1.318(2) | N(1)-C(13)-C(12) | 122.50(16) | |||
| N(4)-C(13) | 1.337(2) | N(1)-C(14)-O(1) | 120.55(15) | |||
| N(3)-C(16) | 1.1150(2) | N(1)-C(14)-C(15) | 124.42(16) | |||
| C(12)-C(13) | 1.422(2) | C(11)-C(15)-C(14) | 119.28(16) | |||
| C(11)-C(12) | 1.406(2) | C(13)-N(1)-C(14) | 117.46(15) | |||
| O(1)-C(14) | 1.338(2) | N(4)-C(13)-N(1) | 116.87(16) | |||
| C(11)-C(15) | 1.404(2) | C(14)-O(1)-C(18) | 119.91(14) | |||
| C(15)-C(14) | 1.417(2) | C(17)-C(12)-C(13) | 116.37(15) | |||
| O(1)-C(18) | 1.466(2) | C(12)-C(11)-C(6) | 120.99(15) | |||
| 4a | ||||||
| N(1)-C(23) | 1.319(3) | N(1)-C(23)-C(22) | 120.62(18) | |||
| C(24)-N(1) | 1.381(3) | N(1)-C(24)-C(25) | 124.48(19) | |||
| C(22)-C(23) | 1.530(3) | N(1)-C(24)-O(1) | 116.55(18) | |||
| C(21)-C(22) | 1.568(3) | C(23)-C(22)-C(21) | 106.96(16) | |||
| C(21)-C(25) | 1.512(3) | C(22)-C(21)-C(25) | 106.41(16) | |||
| C(25)-C(24) | 1.352(3) | C(21)-C(25)-C(24) | 118.82(18) | |||
| C(22)-C(27) | 1.566(3) | N(1)-C(23)-N(2) | 120.29(19) | |||
| C(23)-N(2) | 1.312(3) | N(2)-C(23)-C(22) | 118.99(18) | |||
| C(24)-O(1) | 1.345(2) | C(27)-C (22)-C(26) | 107.67(17) | |||
| 4b | ||||||
| N(1)-C(25) | 1.378(4) | C(24)-C(23)-C(22) | 105.9(3) | |||
| N(1)-C(26) | 1.291(4) | N(4)-C(28)-C(24) | 177.4(4) | |||
| N(2)-C(26) | 1.334(5) | N(2)-C(26)-C(22) | 118.3(3) | |||
| N(3)-C(27) | 1.137(5) | N(3)-C(27)-C(22) | 178.0(4) | |||
| N(4)-C(28) | 1.147(4) | C(26)-C(22)-C(23) | 103.3(3) | |||
| C(22)-C(21) | 1.559(5) | C(24)-C(25)-N(1) | 124.1(3) | |||
| C(22)-C(26) | 1.532(5) | N(2)-C(26)-N(1) | 119.3(3) | |||
| C(23)-C(22) | 1.569(4) | C(21)-C(22)-C(23) | 111.6(3) | |||
| C(24)-C(25) | 1.359(5) | C(26)-N(1)-C(25) | 117.0(3) | |||
| C(23)-H(23) | 0.9800 | C(22)-C(26)-N(1) | 122.4(3) | |||
| C(25)-O(1) | 1.345(4) | O(1)-C(25)-N(1) | 116.8(3) | |||
| C(29)-O(1) | 1.443(5) | C(27)-C(22)-C(23) | 109.9(3) | |||
| C(24)-C(23) | 1.510(5) | C(26)-C(22)-C(23) | 106.3(3) | |||
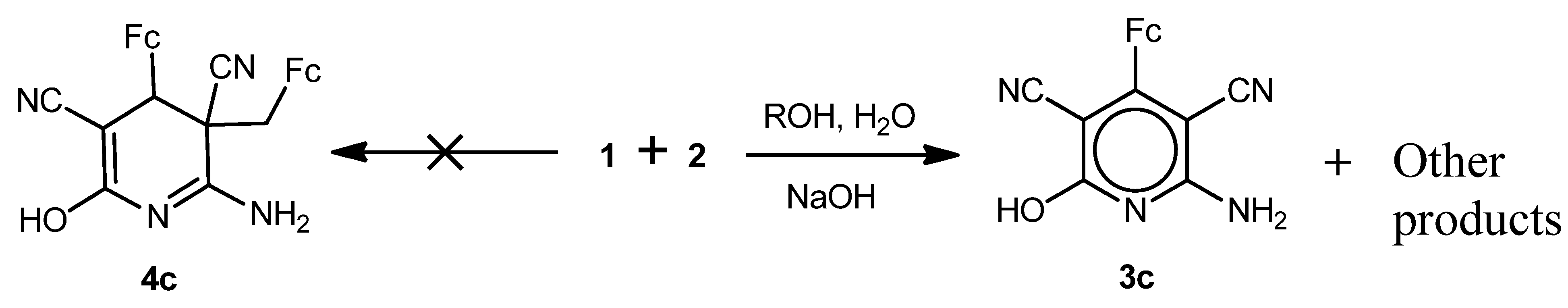



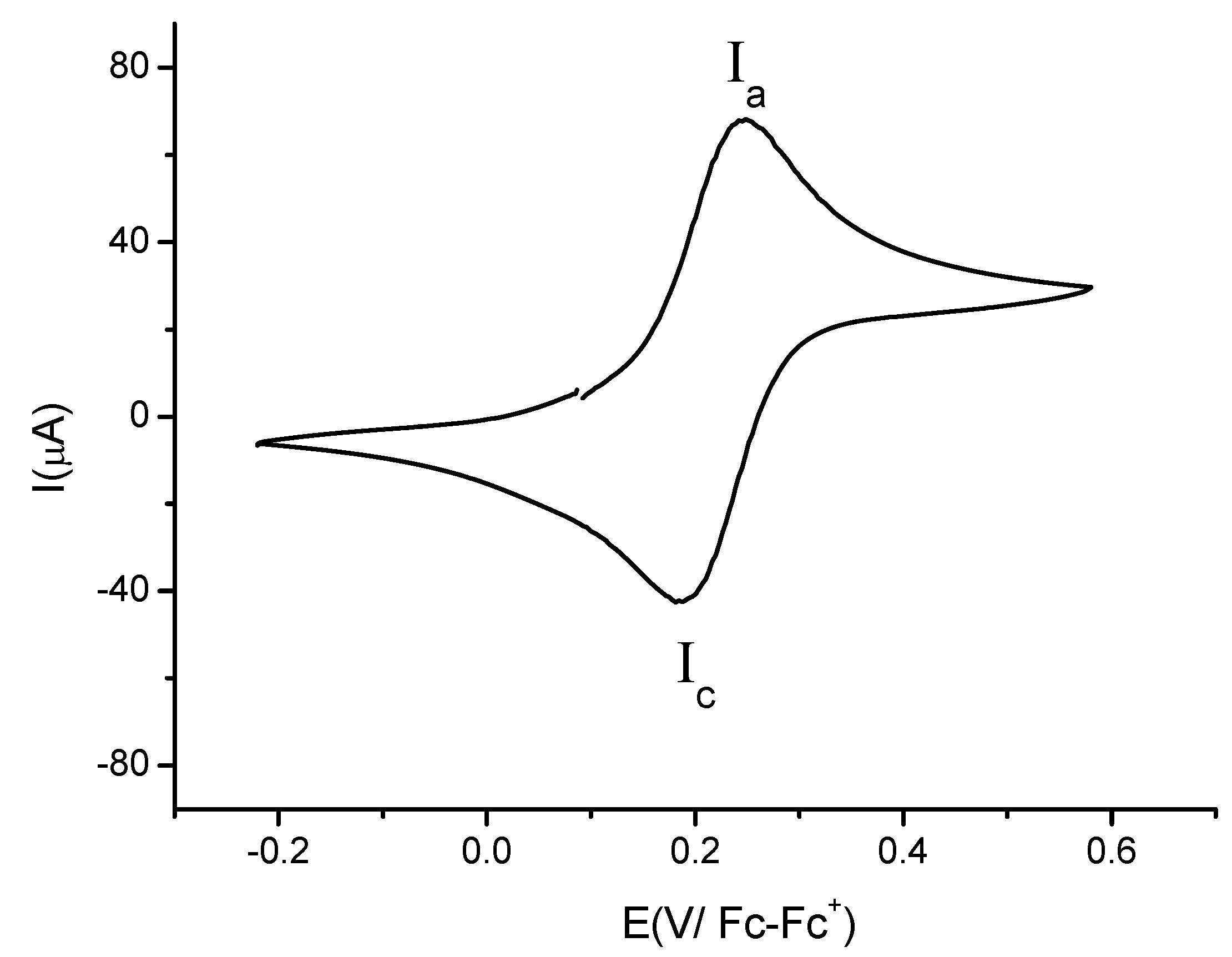
2.2. Electrochemistry

3. Experimental
3.1. General
3.2. Crystal Structures of 3b, 4a and 4b
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles, Structures, Reactions, Synthesis and Applications; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 269–316. [Google Scholar]
- Abdel-Latif, N.A.; Sabry, N.M.; Mohamed, A.M.; Abdulla, M.M. Synthesis, analgesic, and antiparkinsonianprofiles of some pyridine, pyrazoline, and thiopy-rimidine derivatives. Monatsh. Chem. 2007, 138, 715–724. [Google Scholar] [CrossRef]
- Kopf-Maier, P.; Kopf, H. Non-platinum-group metal antitumor agents: History, current status, and perspectives. Chem. Rev. 1987, 87, 1137–1152. [Google Scholar] [CrossRef]
- Miller, T.M.; Ahmed, K.J.; Wrighton, M.S. Complexes of rhenium carbonyl containing ferrocenyl-derived ligands: Tunable electron density at rhenium by control of the redox state of the ferrocenyl ligand. Inorg. Chem. 1989, 28, 2347–2355. [Google Scholar] [CrossRef]
- Rajput, J.; Hutton, A.T.; Moss, J.R.; Su, H.; Imrie, C. Ferrocenyl-nitrogen donor ligands. Synthesis and characterization of Rhodium(I) complexes of ferrocenylpyridine and related ligands. J. Organomet. Chem. 2006, 691, 4573–4588. [Google Scholar]
- Beletskaya, I.P.; Tsvetkov, A.V.; Latyshev, G.V.; Tafeenko, V.A.; Lukashev, N.V. Bis(ferrocenyl)mercury as a source of ferrocenyl moiety in Pd-catalyzed reactions of carbon-carbon bond formation. J. Organomet. Chem. 2001, 637–639, 653–663. [Google Scholar]
- Mamane, V. Metal-catalyzed cross-coupling reactions for ferrocene functionalization: Recent applications in synthesis, material science and asymmetric catalysis. Mini-Rev. Org. Chem. 2008, 5, 303–312. [Google Scholar]
- Schvekhgeimer, M.-G.A. Heterylferrocenes. Russ. Chem. Rev. 1996, 65, 66–69. [Google Scholar]
- Shibata, K.; Katsuyama, I.; Izoe, H.; Matsui, M.; Muramutsu, H. Synthesis of 4,6-disubstituted 2-methylpyridines and their 3-carboxamides. J. Heterocycl. Chem. 1993, 30, 277–281. [Google Scholar]
- Shibata, K.; Katsuyama, I.; Matsui, M.; Muramutsu, H. Synthesis of ferrocenyl-substituted 3-cyano-2-methylpyridines. Bull. Chem. Soc. Jpn. 1990, 63, 3710–3712. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Ji, S.-J.; Shen, Z.-L. An efficient synthesis of ferrocenyl substituted 3-cyanopyridine derivatives under ultrasound irradiation. J. Organomet. Chem. 2006, 691, 1356–1360. [Google Scholar]
- Zhuang, Q.; Jia, R.; Tu, S.; Zhang, J.; Jiang, B.; Zhang, Y.; Yao, C. Green chemistry approach to the synthesis of 2-amino-4-aryl-6-ferrocenylpyridine derivatives by a one-pot reaction in aqueous medium. J. Heterocycl. Chem. 2007, 44, 895–900. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, H.; Guo, H.; Yu, L. Microwave absorbing property of Fe-filled nanotubes synthesized by a practical route. Mat. Sci. Eng. B 2007, 138, 101–104. [Google Scholar]
- Biot, C.; Chavain, N.; Dubar, F.; Pradines, B.; Trivelli, X.; Brocard, J.; Forfar, I.; Dive, D. Structure-activity relation-ships of 4-N-substituted ferroquine analogues: Time to re-evaluate the mechanism of action of ferroquine. J. Organomet. Chem. 2009, 694, 845–854. [Google Scholar] [CrossRef]
- Yao, T.; Rechnitz, G.A. Amperometric enzyme-immunosensor based on ferrocene-mediated amplification. Biosensors 1987, 3, 307–312. [Google Scholar] [CrossRef]
- Epton, R.; Marr, G.; Regers, G.K. The ferrocene analogues of salicylic acid and aspirin. J. Organomet. Chem. 1976, 110, C42–C44. [Google Scholar]
- Biot, C.; Delhaes, L.; N’Diaye, C.M.; Maciejewski, L.A.; Camus, D.; Dive, D. Synthesis and antimalarial activity in vitro of potential metabolites of ferrochloro-quine and related compounds. Bioorg. Med. Chem. 1999, 7, 2843–2847. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—From teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Chavain, N.; Vezin, V.; Dive, D.; Touati, N.; Paul, J.-F.; Buisine, E.; Biot, C. Investigation of the redox behavior of ferroquine, a new antimalarial. Mol. Pharm. 2008, 5, 510–516. [Google Scholar]
- Kaifer, A.E.; de Mendoza, J. Comprehensive Supramolecular Chemistry; Elsevier: Oxford, UK, 1996; Volume 1, pp. 701–725. [Google Scholar]
- Kowalski, K.; Winter, R.F. The synthesis and electrochemistry of 2,5-dimethylazaferrocenes with heteroaryl bridges. J. Organomet. Chem. 2012, 700, 58–68. [Google Scholar] [CrossRef]
- Kowalski, K.; Koceva-Chyla, A.; Pieniazek, K.; Bernasinska, J.; Skiba, J.; Rybarczyk-Pirek, A.J.; Józwiak, Z. The synthesis, structure, electrochemistry and in vitro anticancer activity studies of ferrocenyl-thymine conjugates. J. Organomet. Chem. 2009, 694, 1041–1048. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Guy Orpen, A.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II 1987, S1–S19. [Google Scholar]
- Postnov, V.N.; Klimova, E.I.; Pushin, A.N.; Meleshonkova, N.N. The interaction of the 1,3-bis(p-methoxyphenyl)allylic cation with ferrocenyl-1,3-butadienes. Metalloorg. Chem. 1992, 5, 564–569. [Google Scholar]
- Tietze, L.F.; Brasche, G.; Gerike, K.M. Domino Reactions in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed; John Wiley and Sons: New York, NY, USA, 2001; Chapter 5. [Google Scholar]
- Wright, J.R.; Shaffer, K.J.; McAdam, C.J.; Crowley, J.D. 3,5-Diferrocenylpyridine: Synthesis, characterisation, palladium(II) dichloride complex and electrochemistry. Polyhedron 2012, 36, 73–78. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Zanello, P. Inorganic Electrochemistry, Theory, Practice and Application; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Robin, M.B.; Day, P. Mixed valence chemistry. A survey and classification. Adv. Inorg. Chem. Radiochem. 1967, 10, 247–422. [Google Scholar] [CrossRef]
- Gritzner, G.; Küta, J. Recommendations on reporting electrode potencials in nonaqueous solvents. Pure Appl. Chem. 1984, 4, 461–466. [Google Scholar] [CrossRef]
- Postnov, V.N.; Polivin, Y.N.; Sazonova, V.A. Fragmentation of β-dicarbonyl-compounds with ferrocenyl group. Dokl. Akad. Nauk SSSR 1983, 271, 1402–1404. [Google Scholar]
- Toma, S.; Putala, M.; Salisava, M. Ultrasound-accelerated synthesis of ferrocene-containing pyrimidine derivatives. Collect. Czech. Chem. Commun. 1987, 52, 395–398. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1994. [Google Scholar]
- Sample Availability: Samples of the compounds 3a,b,d and 4a,b are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Klimova, E.I.; Flores-Alamo, M.; Maya, S.C.; Martínez, M.E.; Ortiz-Frade, L.; Klimova, T. 4-Ferrocenylpyridine- and 4-Ferrocenyl-3-ferrocenylmethyl-3,4-dihydropyridine-3,5-dicarbonitriles: Multi-Component Synthesis, Structures and Electrochemistry. Molecules 2012, 17, 10079-10093. https://doi.org/10.3390/molecules170910079
Klimova EI, Flores-Alamo M, Maya SC, Martínez ME, Ortiz-Frade L, Klimova T. 4-Ferrocenylpyridine- and 4-Ferrocenyl-3-ferrocenylmethyl-3,4-dihydropyridine-3,5-dicarbonitriles: Multi-Component Synthesis, Structures and Electrochemistry. Molecules. 2012; 17(9):10079-10093. https://doi.org/10.3390/molecules170910079
Chicago/Turabian StyleKlimova, Elena I., Marcos Flores-Alamo, Sandra Cortez Maya, Mark E. Martínez, Luis Ortiz-Frade, and Tatiana Klimova. 2012. "4-Ferrocenylpyridine- and 4-Ferrocenyl-3-ferrocenylmethyl-3,4-dihydropyridine-3,5-dicarbonitriles: Multi-Component Synthesis, Structures and Electrochemistry" Molecules 17, no. 9: 10079-10093. https://doi.org/10.3390/molecules170910079