Ultrasound Irradiation Promoted Enzymatic Transesterification of (R/S)-1-Chloro-3-(1-naphthyloxy)-2-propanol
Abstract
:1. Introduction
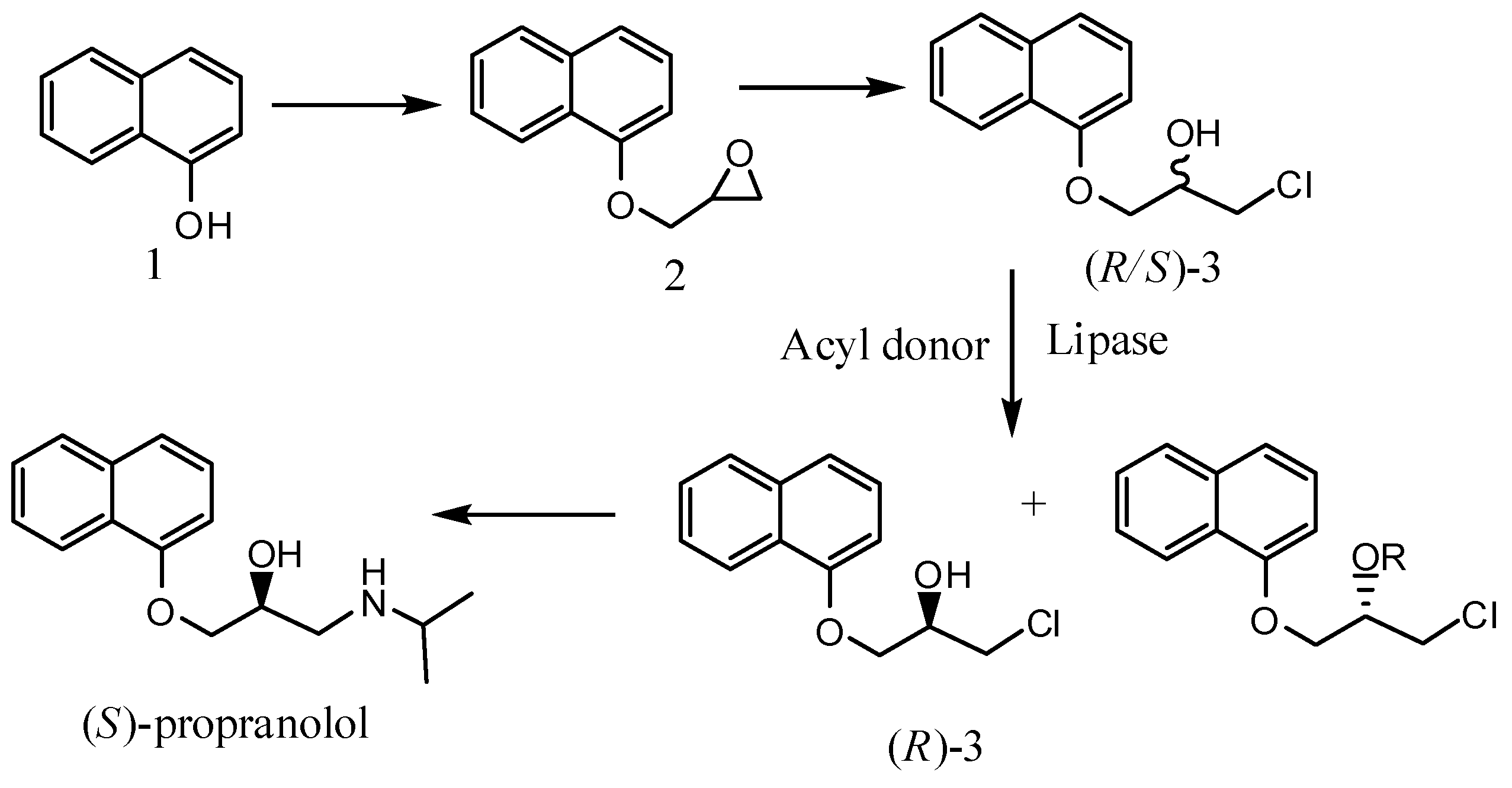
2. Results and Discussion
2.1. Effect of Enzyme Type
| Enzyme | Conventional shaking | Ultrasound irradiation | ||
|---|---|---|---|---|
| Enzyme activity (μmol·g−1·min−1) | Enantioselectivity ( E value) | Enzyme activity (μmol·g−1·min−1) | Enantioselectivity ( E value) | |
| Pseudomonas sp. Lipase (PSL) | 43.3 ± 3.7 | 87 ± 7 | 78.3 ± 3.2 | 98 ± 6 |
| Candida antarctica lipase B (CAL-B) | 59.6 ± 2.3 | 70 ± 6 | 85.6 ± 3.1 | 72 ± 8 |
| Porcine pancreas lipase (PPL) | 23.7 ± 6.8 | 27 ± 4 | 36.7 ± 1.3 | 21 ± 2 |
| Candida sp. 415 lipase (CSL) | 35.6 ± 4.4 | 34 ± 1 | 50.6 ± 2.5 | 36 ± 2 |
| Pseudomonas fluorescens lipase (PFL) | 29.8 ± 5.2 | 29 ± 2 | 41.8 ± 1.8 | 37 ± 3 |
2.2. Effect of Ultrasound Power

2.3. Effect of Solvent
| Solvent | Log P | Enzyme activity (μmol·g−1·min−1) | Enantioselectivity ( E value) |
|---|---|---|---|
| Acetonitrile | −0.33 | 10.9 ± 4.2 | 15 ± 2 |
| Tetrahydrofuran | 0.49 | 28.7 ± 2.4 | 27 ± 4 |
| Cyclohexane | 1.20 | 35.6 ± 3.2 | 53 ± 5 |
| Toluene | 2.50 | 61.4 ± 2.6 | 65 ± 3 |
| Methyl tert-butyl ether | 2.90 | 69.4 ± 1.3 | 90 ± 5 |
| n-Hexane | 3.50 | 78.3 ± 3.2 | 98 ± 6 |
2.4. Effect of Acyl Donor
| Acyl donor | Vinyl acetate | Vinyl propionate | Vinyl butyrate | Vinyl valerate | Vinyl caproate |
|---|---|---|---|---|---|
| Enayzme activity (μmol·g−1·min−1) | 89.6 ± 3.8 | 78.3 ± 3.2 | 66.1 ± 4.1 | 45.2 ± 3.0 | 20.4 ± 2.5 |
| Enantioselectivity ( E value) | 69 ± 2 | 98 ± 6 | 81 ± 3 | 73 ± 4 | 70 ± 5 |
2.5. Effect of Temperature
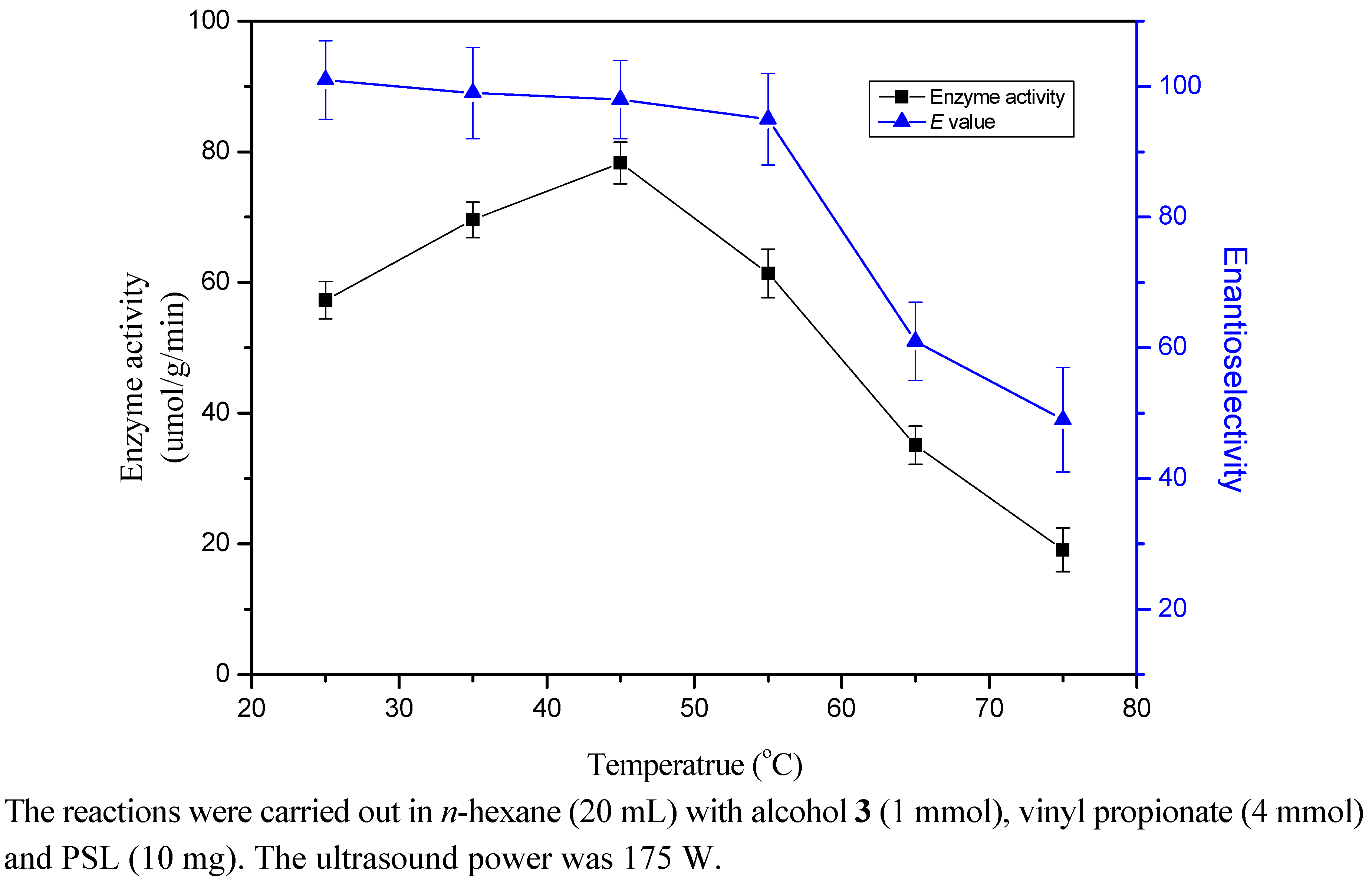
2.6. Substrate Ratio
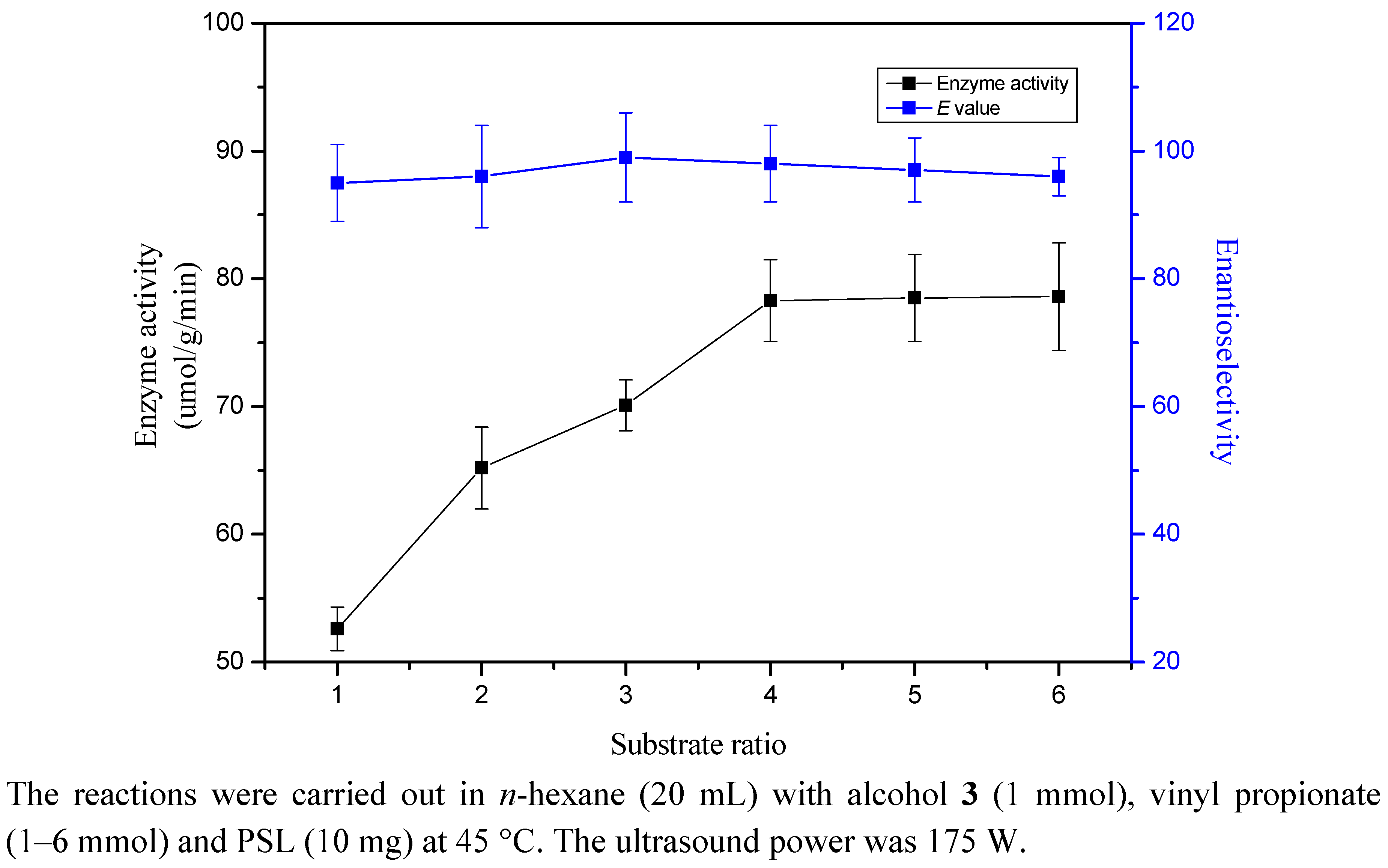
3. Experimental
3.1. Catalysts and Chemicals
3.2. Enantioselective transesterification of (R/S)-1-chloro-3-(1-naphthyloxy)-2-propanol (3)

3.3. Analytical Methods
4. Conclusions
Acknowledgments
References
- Kumar, P.; Naidu, V.; Gupta, P. Application of hydrolytic kinetic resolution (HKR) in the synthesis of bioactive compounds. Tetrahedron 2007, 63, 2745–2785. [Google Scholar] [CrossRef]
- Schaus, S.E.; Brandes, B.D.; Larrow, J.F.; Tokunaga, M.; Hansen, K.B.; Gould, A.E.; Furrow, M.E.; Jacobsen, E.N. Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)CoIII Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc. 2002, 124, 1307–1315. [Google Scholar]
- Wasson, B.K.; Gibson, W.K.; Stuart, R.S.; Williams, H.W.R.; Yates, C.H. beta-Adrenergic blocking agents. 3-(3-Substituted-amino-2-hydroxypropoxy)-4-substituted-1,2,5-thiadiazoles. J. Med. Chem. 1972, 15, 651–655. [Google Scholar] [CrossRef]
- Patel, R.N.; Banerjee, A.; Ko, R.Y.; Howell, J.M.; Li, W.S.; Comezoglu, F.T.; Partyka, R.A.; Szarka, L.J. Enzymatic Preparation of (3R-cis)-3-(acetyloxy)-4-Phenyl-2-Azetidinone: A Taxol Side-Chain Synthon. Biotechnol. Appl. Biochem. 1994, 20, 23–33. [Google Scholar]
- Patel, R.N.; Liu, M.; Banerjee, A.; Szarka, L.J. Stereoselective Enzymatic Hydrolysis of (exo,exo)-7-Zoxabicyclo[2.2.1]Heptane-2,3-Dimethanol Diacetate Ester in a Biphasic System. Appl. Microbiol. Biotechnol. 1992, 37, 180–183. [Google Scholar]
- Patel, R.N.; Howell, J.M.; Banerjee, A.; Fortney, K.F.; Szarka, L.J. Stereoselective Enzymatic Esterification of 3-Benzoylthio-2-Methylpropanoic. Appl. Microbiol. Biotechnol. 1991, 36, 29–34. [Google Scholar] [CrossRef]
- Chiou, T.W.; Chang, C.C.; Lai, C.T.; Tai, D.F. Kineticresolution of propranolol by a lipase-catalyzed N-acetylation. Bioorg. Med. Chem. Lett. 1997, 7, 433–436. [Google Scholar] [CrossRef]
- Oveimar, B.; Cesar, A.; Claudia, O.; Rodrigo, T. Kineticresolution of (R/S)-propranolol (1-isopropylamino-3-(1-naphtoxy)-2-propanolol) catalyzed by immobilized preparations of Candida antarctica lipase B (CAL-B). N. Biotechnol. 2010, 27, 844–850. [Google Scholar] [CrossRef]
- Bevinakatti, H.S.; Banerji, A.A. Practical Chemoenzymatic Synthesis of Both Enantiomers of Propranolol. J. Org. Chem. 1991, 56, 5372–5375. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature 1991, 351, 491–494. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Li, C.Y.; Wang, P.; Wang, Z.; Tang, J.; Wang, L. Improvement of the enantioselectivity and activity of lipase from Pseudomonas sp. via adsorption on a hydrophobic support: kinetic resolution of 2-octanol. Biocatal. Biotransformation 2009, 27, 340–347. [Google Scholar] [CrossRef]
- Fernández-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Palomo, J.M.; Segura, R.L.; Fernández-Lorente, G.; Guisán, J.M.; Fernández-Lafuente, R. Glutaraldehyde modification of lipases adsorbed on aminated supports: A simple way to improve their behaviour as enantioselective biocatalyst. Enzyme Microb. Technol. 2007, 40, 704–707. [Google Scholar] [CrossRef]
- Palomo, J.M.; Segura, R.L.; Fernández-Lorente, G.; Guisán, J.M.; Fernández-Lafuente, R. Enzymatic resolution of (±)-glycidyl butyrate in aqueous media. Strong modulation of the properties of the lipase from Rhizopus oryzae via immobilization techniques. Tetrahedron: Asymmetry 2004, 15, 1157–1161. [Google Scholar] [CrossRef]
- Lee, J.; Snyder, J.K. Ultrasound-promoted diels-alder reaction: syntheses of tanshinone IA, nortanshinone, and (±)-tanshindiol B. J. Am. Chem. Soc. 1989, 111, 1522–1524. [Google Scholar] [CrossRef]
- Pizzuti, L.; Piovesan, L.A.; Flores, A.F.; Quina, F.H.; Pereira, C.M. Environmentally friendly sonocatalysis promoted preparation of 1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-1H-pyrazoles. Ultrason. Sonochem. 2009, 16, 728–731. [Google Scholar] [CrossRef]
- Pizzuti, L.; Martins, P.L.; Ribeiro, B.A.; Quina, F.H.; Pinto, E.; Flores, A.F.; Venzke, D.; Pereira, C.M. Efficient sonochemical synthesis of novel 3,5-diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Ultrason. Sonochem. 2010, 17, 34–37. [Google Scholar] [CrossRef]
- Gogate, P.R.; Kabadi, A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Matsud, T.; Kanamaru, R.; Watanabe, K.; Kamitanaka, T.; Harada, T.; Nakamura, K. Control of enantioselectivity of lipase-catalyzed esterification in supercritical carbon dioxide by tuning the pressure and temperature. Tetrahedron: Asymmetry 2003, 14, 2087–2091. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Tian, J.; Zhao, B.; Wei, X.F.; Su, Y.L.; Li, C.Y.; Cao, S.G.; Ji, T.F.; Wang, L. The effect of ultrasound on lipase-catalyzed regioselective acylation of mangiferin in non-aqueous solvents. J. Asian Nat. Prod. Res. 2010, 12, 56–63. [Google Scholar] [CrossRef]
- Braginskaya, F.I.; Zaitzeva, E.A.; Zorina, O.M.; Poltorak, O.M.; Chukrai, E.S.; Dunn, F. Low intensity ultrasonic effects on yeast hexokinase. Radiat. Environ. Biophys. 1990, 29, 47–56. [Google Scholar] [CrossRef]
- Dong, H.; Gao, S.J.; Han, S.P.; Cao, S.G. Purification and characterization of a Pseudomonas sp. Lipase and its properties in non-aqueous media. Biotechnol. Appl. Biochem. 1999, 30, 251–256. [Google Scholar]
- Sakakibara, M.; Wang, D.; Takahashi, R.; Takahashi, K.; Mori, S. Influence of ultrasound irradiation on hydrolysis of sucrose catalyzed by invertase. Enzyme. Microb. Technol. 1996, 18, 444–448. [Google Scholar] [CrossRef]
- An, B.Y.; Xie, X.N.; Xun, E.N.; Wang, J.X.; Wang, R.; Sun, R.X.; Wang, L.; Wang, Z. Ultrasound-promoted lipase-catalyzed enantioselective transesterification of (R,S)-glycidol. Chem. Res. Chin. Univ. 2011, 27, 845–849. [Google Scholar]
- Ozbek, B.; Ülgen, K.Ö. The stability of enzymes after sonication. Proc. Biochem. 2000, 35, 1037–1043. [Google Scholar] [CrossRef]
- Manjón, A.; Iborra, J.L.; Arocas, A. Short-chain flavour ester synthesis by immobilized lipase in organic media. Biotechnol. Lett. 1991, 5, 339–344. [Google Scholar]
- Xiao, H.P.; Li, Z.Y.; Ward, O.P. Hydrolysis of ethyl mandelate and esterification of 2-bromopropionic acid in micro-emulsions. J. Ind. Microbiol. Biotechnol. 1995, 14, 416–419. [Google Scholar]
- Ozaki, S.; Yamashita, K.; Konishi, T.; Maekawa, T.; Eshima, M.; Uemura, A.; Ling, L. Enzyme Aided Regioselective Acylation of Nucleosides. Nucleos. Nucleot. 1995, 14, 401–404. [Google Scholar] [CrossRef]
- Karl, H.; Torbjarn, N. Enantioselectivity of some lipases: Control and prediction. Pure Appl. Chem. 1992, 64, 1129–1134. [Google Scholar] [CrossRef]
- Sakai, T.; Kishimoto, T.; Tanaka, Y.; Ema, T.; Utaka, M. Low-temperature method for enhancement of enantioselectivity in the lipase-catalyzed kinetic resolutions of solketal and some chiral alcohols. Tetrahedron Lett. 1998, 39, 7881–7884. [Google Scholar] [CrossRef]
- Phillips, R.S. Temperature modulation of the stereochemistry of enzymatic catalysis: Prospects for exploitation. Trends Biotechnol. 1996, 14, 13–16. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar]
- Mateo, C.; Palomo, J.M.; Fernández-Lorente, G.; Guisán, J.M.; Fernández-Lafuente, R. Enzyme Microb. Technol. 2007, 40, 1451–1463. [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, F.; Zhang, H.; Wang, J.; Chen, G.; Fang, X.; Wang, Z.; Wang, L. Ultrasound Irradiation Promoted Enzymatic Transesterification of (R/S)-1-Chloro-3-(1-naphthyloxy)-2-propanol. Molecules 2012, 17, 10864-10874. https://doi.org/10.3390/molecules170910864
Wang F, Zhang H, Wang J, Chen G, Fang X, Wang Z, Wang L. Ultrasound Irradiation Promoted Enzymatic Transesterification of (R/S)-1-Chloro-3-(1-naphthyloxy)-2-propanol. Molecules. 2012; 17(9):10864-10874. https://doi.org/10.3390/molecules170910864
Chicago/Turabian StyleWang, Feng, Hong Zhang, Jiaxin Wang, Ge Chen, Xuedong Fang, Zhi Wang, and Lei Wang. 2012. "Ultrasound Irradiation Promoted Enzymatic Transesterification of (R/S)-1-Chloro-3-(1-naphthyloxy)-2-propanol" Molecules 17, no. 9: 10864-10874. https://doi.org/10.3390/molecules170910864




