Chemical Composition of Hexane Extract of Citrus aurantifolia and Anti-Mycobacterium tuberculosis Activity of Some of Its Constituents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Characterization of C. aurantifolia Constituents

2.2. GC-MS Analysis
| Peak | Compound | RT a | RI b | % c |
|---|---|---|---|---|
| 1 | Tetrahydro-2-methyl-2 H-pyran | 5.25 | 838 | 0.72 |
| 2 | 4-Hexen-3-one | 5.54 | 855 | 0.51 |
| 3 | 3-Methyl-3-penten-2-one | 5.61 | 859 | 0.33 |
| 4 | 3-Hexen-2-one | 5.67 | 862 | 0.48 |
| 5 | 2,3-Dimethyl-2,3-butanediol | 6.30 | 898 | 1.67 |
| 6 | Resorcinol | 8.35 | 1016 | 3.65 |
| 7 | p-Cymene | 8.85 | 1046 | 0.36 |
| 8 | 1-Methoxycyclohexene | 9.56 | 1089 | 8.00 |
| 9 | Linalool oxide | 9.97 | 1115 | 1.18 |
| 10 | Crysantenile acetate | 10.60 | 1156 | 0.40 |
| 11 | Corylone | 10.92 | 1177 | 6.93 |
| 12 | Terpinen-4-ol | 11.47 | 1213 | 1.66 |
| 13 | α-Terpineol | 11.74 | 1232 | 5.97 |
| 14 | 3-Methyl-1,2-cyclopentanedione | 12.10 | 1257 | 8.27 |
| 15 | 3,7-Dimethyl-(Z)-2,6-octadienal | 12.36 | 1276 | 1.09 |
| 16 | Carvone | 12.49 | 1284 | 0.88 |
| 17 | Geraniol | 12.60 | 1292 | 1.15 |
| 18 | Citral | 12.77 | 1305 | 2.21 |
| 19 | 1,8-Dimethyl-4-(1-methylethyl)-spiro[4.5]dec-8-en-7-one | 12.96 | 1318 | 0.56 |
| 20 | Geranyl formate | 13.11 | 1329 | 0.70 |
| 21 | Oleic acid | 13.93 | 1390 | 0.69 |
| 22 | 7-Methyl-(Z)-8-tetradecen-1-ol acetate | 14.20 | 1410 | 2.83 |
| 23 | Geranyl acetone | 14.78 | 1455 | 1.84 |
| 24 | Bergamotene | 14.96 | 1470 | 1.00 |
| 25 | (Z)-8-Methyl-9-tetradecenoic acid | 15.28 | 1494 | 1.24 |
| 26 | trans-α-Bisabolene | 15.89 | 1545 | 1.02 |
| 27 | Caryophyllene oxide | 17.05 | 1643 | 3.02 |
| 28 | Spathulenol | 17.60 | 1691 | 1.95 |
| 29 | Umbelliferone | 19.06 | 1828 | 4.36 |
| 30 | (Z)-11(13,14-Epoxy)tetradecen-1-ol acetate | 19.29 | 1849 | 0.59 |
| 31 | trans-Phytol | 19.52 | 1872 | 0.22 |
| 32 | 1-Heptatriacontanol | 19.65 | 1884 | 0.42 |
| 33 | Versalide | 20.08 | 1926 | 0.51 |
| 34 | Methyl palmitate | 20.45 | 1964 | 0.29 |
| 35 | Palmitic acid | 21.19 | 2031 | 6.89 |
| 36 | 5,7-Dimethoxycoumarin | 21.83 | 2083 | 15.80 |
| 37 | 5-Methoxypsoralen | 22.55 | 2154 | 1.14 |
| 38 | Linoleic acid | 22.77 | 2179 | 0.96 |
| 39 | Tricosane | 23.88 | 2305 | 0.31 |
| 40 | 5,8-Dimethoxypsoralen | 24.12 | 2332 | 6.08 |
| 41 | Pentacosane | 25.86 | 2506 | 0.46 |
| 42 | Tetracosanal | 27.80 | 2650 | 0.70 |
| 43 | Octacosane | 28.78 | 2711 | 0.39 |
| 44 | Nonacosane | 33.40 | 2915 | 0.50 |
2.3. Antimycobacterial Activity of Constituents from C. aurantifolia
| Compound | H37Rv a | H10 b | M15 c | M26 d |
|---|---|---|---|---|
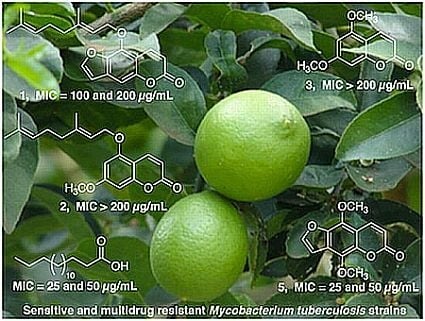
| 5-Geranyloxypsoralen (1) | 50 | 200 | 100 | 100 |
| 5-Geranyloxy-7-methoxycoumarin (2) | 200 | 200 | 100 | 100 |
| 5,7-Dimethoxycoumarin (3) | >200 | NT | NT | NT |
| 5,8-Dimethoxypsoralen (5) | 25 | 25 | >50 | 50 |
| 4-Hexen-3-one | 50 | >200 | >200 | >200 |
| 3-Methyl-3-penten-2-one | >200 | NT | NT | NT |
| Resorcinol | >200 | NT | NT | NT |
| p-Cymene | >200 | NT | NT | NT |
| Linalool oxide | >200 | NT | NT | NT |
| Terpinen-4-ol | 200 | >200 | >200 | >200 |
| 3-Methyl-1,2-cyclopentanedione | >200 | NT | NT | NT |
| Carvone | 200 | >200 | >200 | >200 |
| Geraniol | 200 | >200 | >200 | >200 |
| Citral | 50 | >200 | >200 | 200 |
| Geranyl formate | >200 | NT | NT | NT |
| Oleic acid | 100 | 100 | 100 | 100 |
| Methyl palmitate | >200 | NT | NT | NT |
| Palmitic acid | 25 | 50 | 50 | 50 |
| Linoleic acid | 50 | 100 | 100 | 100 |
| Pinacol | >200 | NT | NT | NT |
| Ethambutol | 2.0 | 15 | 15 | 15 |
| Isoniazid | 0.02 | 5 | 7 | 6 |
| Rifampicin | 0.08 | 9 | 10 | 12 |
3. Experimental
3.1. General Experimental Procedures
3.2. Chemicals
3.3. Mycobacterium Tuberculosis Strains
3.4. Plant Material
3.5. Extraction and Isolation of Constituents from C. aurantifolia
3.6. Spectroscopical Data
3.7. GC-MS Analysis
3.8. Antimycobacterial Activity
4. Conclusions
Acknowledgments
Conflict of Interest
References
- World Health Organization Home Page. Available online: http://www.who.int/tb/publications/2011/factsheet_tb_2011.pdf (accessed on 20 July 2012).
- Tripathi, R.P.; Tewari, N.; Dwivedi, N.; Tiwari, V.K. Fighting tuberculosis: An old disease with new challenges. Med. Res. Rev. 2005, 25, 93–131. [Google Scholar] [CrossRef]
- Copp, B.R. Antimycobacterial natural products. Nat. Prod. Rep. 2003, 20, 535–557. [Google Scholar] [CrossRef]
- Okunade, A.L.; Elvin-Lewis, M.P.F.; Lewis, W.H. Natural antimycobacterial metabolites: Current status. Phytochemistry 2004, 65, 1017–1032. [Google Scholar]
- Nayyar, A.; Jain, R. Recent advances in new structural classes of anti-tuberculosis agents. Curr. Med. Chem. 2005, 12, 1873–1886. [Google Scholar] [CrossRef]
- Copp, B.R.; Pearce, A.N. Natural product growth inhibitors of Mycobacterium tuberculosis. Nat. Prod. Rep. 2007, 24, 278–297. [Google Scholar] [CrossRef]
- García, A.; Bocanegra-García, V.; Palma-Nicolás, J.P.; Rivera, G. Recent advances in antitubercular natural products. Eur. J. Med. Chem. 2012, 49, 1–23. [Google Scholar] [CrossRef]
- Camacho-Corona, M.R.; Ramírez-Cabrera, M.A.; González-Santiago, O.; Garza-González, E.; Palacios, I.P.; Luna-Herrera, J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother. Res. 2008, 22, 82–85. [Google Scholar] [CrossRef]
- Camacho-Corona, M.R.; Favela-Hernández, J.M.J.; González-Santiago, O.; Garza-González, E.; Molina-Salinas, G.M.; Said-Fernández, S.; Delgado, G.; Luna-Herrera, J. Evaluation of some plant-derived secondary metabolites against sensitive and multidrug-resistant Mycobacterium tuberculosis. J. Mex. Chem. Soc. 2009, 53, 71–75. [Google Scholar]
- Favela-Hernández, J.M.J.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012. [Google Scholar] [CrossRef]
- Esquivel-Ferriño, P.C.; Favela-Hernández, J.M.; Garza-González, E.; Waksman, N.; Ríos, M.Y.; Camacho-Corona, M.R. Antimycobacterial activity of constituents from Foeniculum vulgare var. dulce grown in México. Molecules 2012, 17, 8471–8482. [Google Scholar]
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Morton, J. Mexican Lime. In Fruits of Warm Climates, 1st ed; J.F. Morton: Miami, FL, USA, 1987; pp. 168–172. [Google Scholar]
- Apraj, V.; Thakur, N.D.; Bhagwat, A.; Mallya, R.; Sawant, L.; Pandita, N. Pharmacognostic and phytochemical evaluation of Citrus aurantifolia (Christm) Swingle peel. Pharmacogn. J. 2011, 3, 70–76. [Google Scholar] [CrossRef]
- Feger, W.; Brandauer, H.; Ziegler, H. Sesquiterpene hydrocarbons of cold-pressed lime oils. Flav. Frag. J. 2000, 15, 281–284. [Google Scholar] [CrossRef]
- Johann, S.; Smania, A.; Pizzolatti, M.G.; Schripsema, J.; Braz-Filho, R.; Branco, A. Complete 1H and 13C-NMR assignments and antifungal activity of two 8-hydroxy flavonoids in mixture. An. Acad. Bras. Cienc. 2007, 79, 215–222. [Google Scholar]
- Piccinelli, A.L.; Garcia, M.M.; Armenteros, D.M.; Alfonso, M.A.; Arevalo, A.C.; Campone, L.; Rastrelli, L. HPLC-PDA-MS and NMR characterization of C-glycosyl flavones in a hydroalcoholic extract of Citrus aurantifolia leaves with antiplatelet activity. J. Agric. Food. Chem. 2008, 56, 1574–1581. [Google Scholar]
- Jiwajinda, S.; Santisopasri, V.; Ohigashi, H. Coumarin-related compounds as plant growth inhibitors from two rutaceous plants in thailand. Biosci. Biotechnol. Biochem. 2000, 64, 420–423. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Asekun, O.T. Comparative study of the chemical profiles of the essential oils of ripe and rotten fruits of Citrus aurantifolia Swingle. Nat. Prod. Commun. 2008, 3, 1133–1136. [Google Scholar]
- Chisholm, G.M.; Wilson, M.A.; Gaskey, G.M. Characterization of aroma volatiles in the key lime essential oils (Citrus aurantifolia Swingle). Flav. Frag. J. 2003, 18, 106–115. [Google Scholar] [CrossRef]
- Jafari, S.; Esfahani, S.; Fazeli, M.R.; Jamalifar, H.; Samadi, M.; Samadi, N.; Najarian-Toosi, A.; Shams-Ardekani, M.R.; Khanavi, M. Antimicrobial activity of lime essential oil against food-borne pathogens isolated from cream-filled cakes and pastries. Int. J. Biol. Chem. 2011, 5, 258–265. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. peel essential oils. J. Food. Sci. 2012, 77, H40–H46. [Google Scholar]
- Taur, D.J.; Kulkarni, V.B.; Patil, R.Y.; Patil, R.N. Anthelmintic activity of Ocimum sanctum and Citrus aurantifolia oils. Pharmacologyonline 2009, 3, 495–499. [Google Scholar]
- Gharagozloo, M.; Doroudchi, M.; Ghaderi, A. Effects of Citrus aurantifolia concentrated extract on the spontaneous proliferation of MDA-MB-453 and RPMI-8866 tumor cell lines. Phytomedicine 2002, 9, 475–477. [Google Scholar] [CrossRef]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Ahmed, H.H.; Basoudan, N. Protective effect of Citrus sinensis and Citrus aurantifolia against osteoporosis and their phytochemical constituents. J. Med. Plants Res. 2011, 5, 579–588. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Isolation of furanocoumarins from bergamot fruits as HL-60 differentiation-inducing compounds. J. Agric. Food Chem. 1999, 47, 4073–4078. [Google Scholar] [CrossRef]
- Miyake, Y.; Hiramitsu, M. Isolation and extraction of antimicrobial substances against oral bacteria from lemon peel. J. Food Sci. Technol. 2011, 48, 635–639. [Google Scholar] [CrossRef]
- Shiota, H. Volatile components in the peel oil from fingered citron (Citrus medica L. var. sarcodactylis Swingle). Flav. Frag. J. 1990, 5, 33–37. [Google Scholar]
- Hongwei, Y.; Bogang, L.; Changsong, L.; Guolin, Z. Chemical Study on Evodia vestita. Chin. J. Appl. Environ. Biol. 2010, 16, 72–75. [Google Scholar] [CrossRef]
- Miyazawa, M.; Tsukamoto, T.; Anzai, J.; Ishikawa, Y. Insecticidal effect of phthalides and furanocoumarins from Angelica acutiloba against Drosophila melanogaster. J. Agric. Food Chem. 2004, 52, 4401–4405. [Google Scholar] [CrossRef]
- Evans, F.E.; Fu, P.P.; Cairns, T. Long-range coupling constants for structural analysis of complex polycyclic aromatic hydrocarbons by high-field proton magnetic resonance spectrometry. Anal. Chem. 1981, 53, 558–560. [Google Scholar] [CrossRef]
- Craske, J.D.; Suryadi, N.; Wootton, M.A. Comparison of the peel oil components of Australian native lime (Microcitrus australe) and Mexican lime (C. aurantifolia Swingle). J. Sci. Food Agric. 2005, 85, 522–525. [Google Scholar]
- Chamblee, T.S.; Clark, B.C. Analysis and chemistry of distilled lime oil (Citrus aurantifolia Swingle). J. Essent. Oil Res. 1997, 9, 267–274. [Google Scholar]
- Bourgaud, E.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unraveled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Ngwendson, J.N.; Bedir, E.; Efange, S.M.; Okunji, C.O.; Iwu, M.M.; Schuster, B.G.; Khan, I.A. Constituents of Peucedanum zenkeri seeds and their antimicrobial effects. Pharmazie 2003, 58, 587–589. [Google Scholar]
- Abbaskhan, A.; Choudhary, M.I.; Ghayur, M.N.; Parween, Z.; Shaheen, F.; Gilani, A.U.; Maruyama, T.; Iqbal, K.; Tsuda, Y. Biological activities of Indian celery, Seseli diffusum (Roxb. Ex Sm.) Sant. & Wagh. Phytother. Res. 2012, 26, 783–786. [Google Scholar]
- Guo, S.; Li, S.; Peng, Z.; Ren, X. Isolation and identification of active constituents of Toddalia asiatica in cardiovascular system. Zhong Yao Cai 1998, 21, 515–516. [Google Scholar]
- Kleiner, H.E.; Vulimiri, S.V.; Starost, M.F.; Reed, M.J.; DiGiovanni, J. Oral administration of the citrus coumarin, isopimpinellin, block DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz(a)anthracene in SECAR mice. Carcinogenesis 2002, 23, 1667–1675. [Google Scholar] [CrossRef]
- Chiou, W.F.; Huang, Y.L.; Chen, C.F.; Chen, C.C. Vasorelaxing effect of coumarins from Cnidium monnieri on rabbit corpus cavernosum. Planta Med. 2001, 67, 282–284. [Google Scholar] [CrossRef]
- Hudson, J.B.; Miki, N.; Towers, G.H. Isopimpinellin is not phototoxic to viruses and cells. Planta Med. 1987, 53, 306–307. [Google Scholar] [CrossRef]
- Ivie, G.W.; Beier, R.C. Isopimpinellin is not phototoxic in a chick skin assay. Photochem. Photobiol. 1996, 63, 306–307. [Google Scholar] [CrossRef]
- Olguín-Reyes, S.; Camacho-Carranza, R.; Hernández-Ojeda, S.; Elinos-Baez, M.; Espinosa-Aguirre, J.J. Bergamottin is a competitive inhibitor of CYP1A1 and is antimutagenic in the Ames test. Food Chem. Toxicol. 2012, 50, 3094–3099. [Google Scholar] [CrossRef]
- Miyake, Y.; Murakami, A.; Sugiyama, Y.; Isobe, M.; Koshimizu, K.; Ohigashi, H. Identification of coumarins from lemon fruit (Citrus limon) as inhibitors of in vitro tumor promotion and superoxide and nitric oxide generation. J. Agric. Food Chem. 1999, 47, 3151–3157. [Google Scholar] [CrossRef]
- Saravanakumar, D.E.M.; Folb, P.I.; Campbell, B.W.; Smith, P. Antimycobacterial activity of the red alga Polysiphonia virgata. Pharm. Biol. 2008, 46, 254–260. [Google Scholar] [CrossRef]
- Hirsch, I.W.; Barchet, H.M. Significance of medium lengh fatty acids and their salts in the prophylaxis of tuberculosis. Deutsche Zeitschrift fuer Verdauung_und Stoffwechselkrankheiten. 1955, 15, 214–218. [Google Scholar]
- Zhing, C.J.; Yoo, J.S.; Lee, T.G.; Cho, H.Y.; Kim, Y.H.; Kim, W.G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry, 4th ed; Allured Business Media: Carol Stream, IL, USA, 2007; pp. 1–804. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sandoval-Montemayor, N.E.; García, A.; Elizondo-Treviño, E.; Garza-González, E.; Alvarez, L.; Del Rayo Camacho-Corona, M. Chemical Composition of Hexane Extract of Citrus aurantifolia and Anti-Mycobacterium tuberculosis Activity of Some of Its Constituents. Molecules 2012, 17, 11173-11184. https://doi.org/10.3390/molecules170911173
Sandoval-Montemayor NE, García A, Elizondo-Treviño E, Garza-González E, Alvarez L, Del Rayo Camacho-Corona M. Chemical Composition of Hexane Extract of Citrus aurantifolia and Anti-Mycobacterium tuberculosis Activity of Some of Its Constituents. Molecules. 2012; 17(9):11173-11184. https://doi.org/10.3390/molecules170911173
Chicago/Turabian StyleSandoval-Montemayor, Nallely E., Abraham García, Elizabeth Elizondo-Treviño, Elvira Garza-González, Laura Alvarez, and María Del Rayo Camacho-Corona. 2012. "Chemical Composition of Hexane Extract of Citrus aurantifolia and Anti-Mycobacterium tuberculosis Activity of Some of Its Constituents" Molecules 17, no. 9: 11173-11184. https://doi.org/10.3390/molecules170911173