Total Flavonoids of Scutellaria barbata Inhibit Invasion of Hepatocarcinoma via MMP/TIMP in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. TF-SB Inhibits Proliferation in MHCC97H Cells

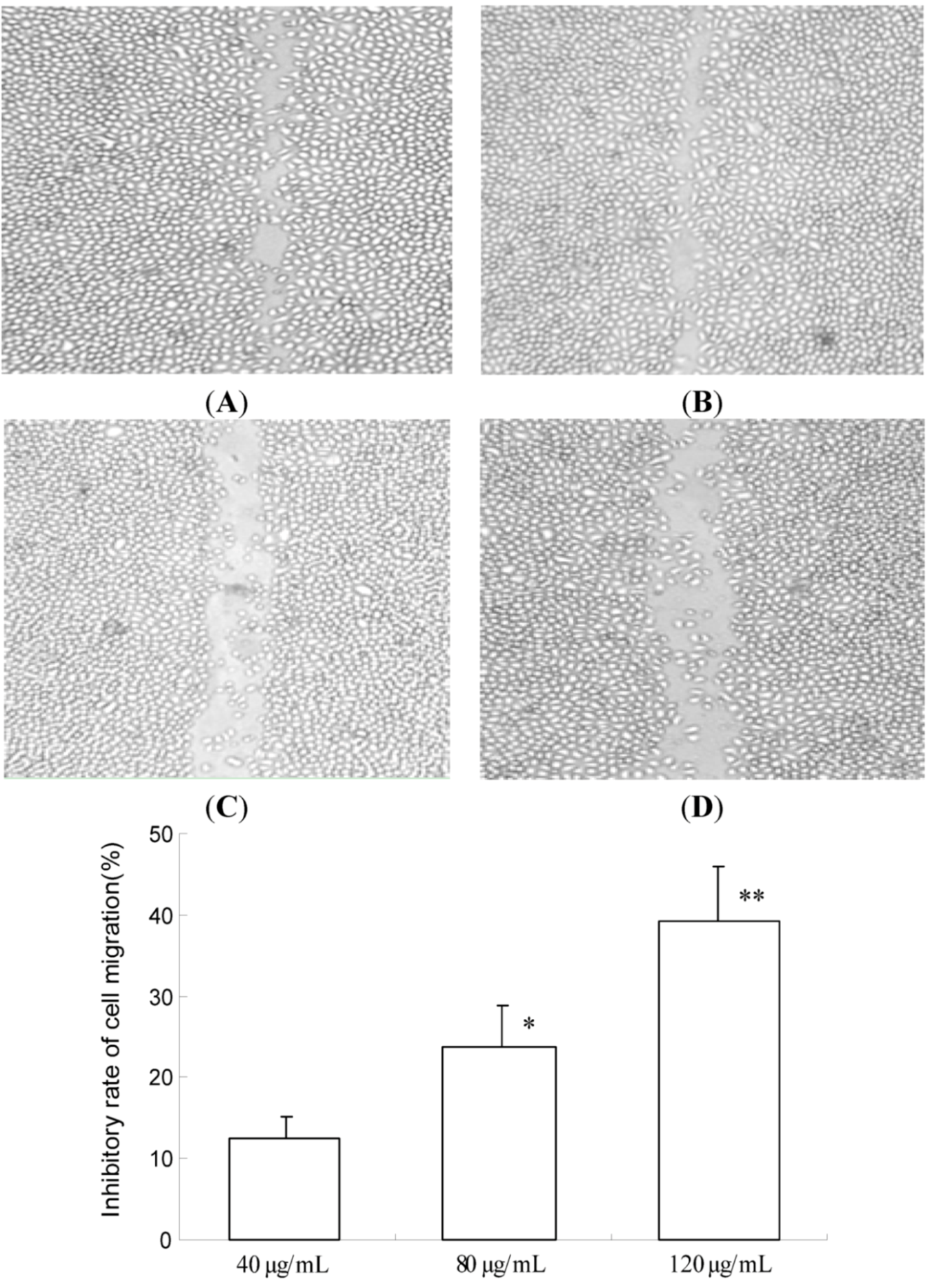
2.2. TF-SB Inhibits Migration and Invasion of MHCC97H Cells

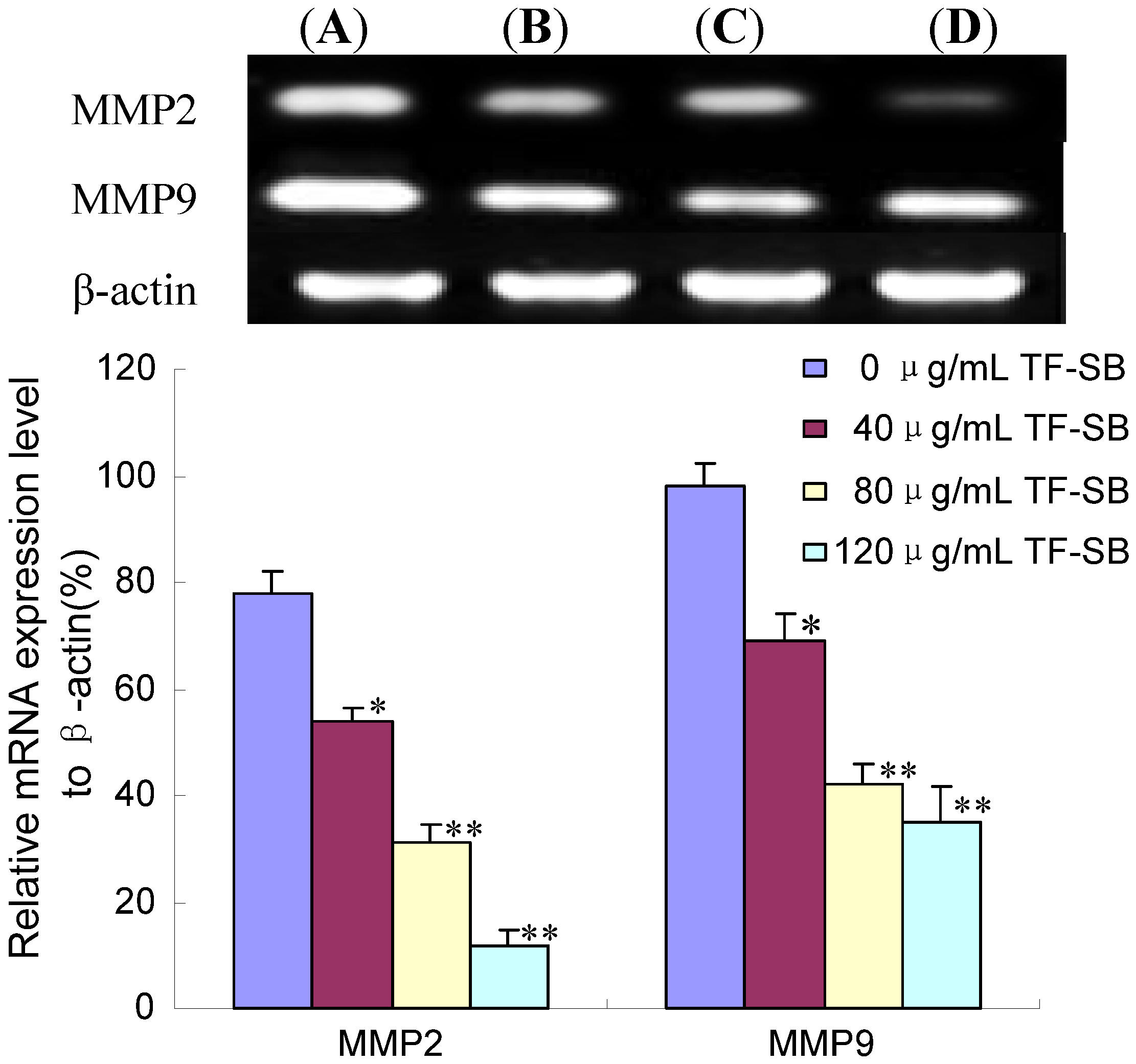
2.3. TF-SB Reduces MMP-2 and MMP-9 mRNA Expression in MHCC97H Cells with RT-PCR Assay
2.4. TF-SB Decreased MMP-2 and MMP-9 Protein Expression with Immunofluorescence and Western Blot Analysis

2.5. TF-SB Promotes TIMP-1 and TIMP-2 mRNA Expression in MHCC97H Cells with RT-PCR Assay

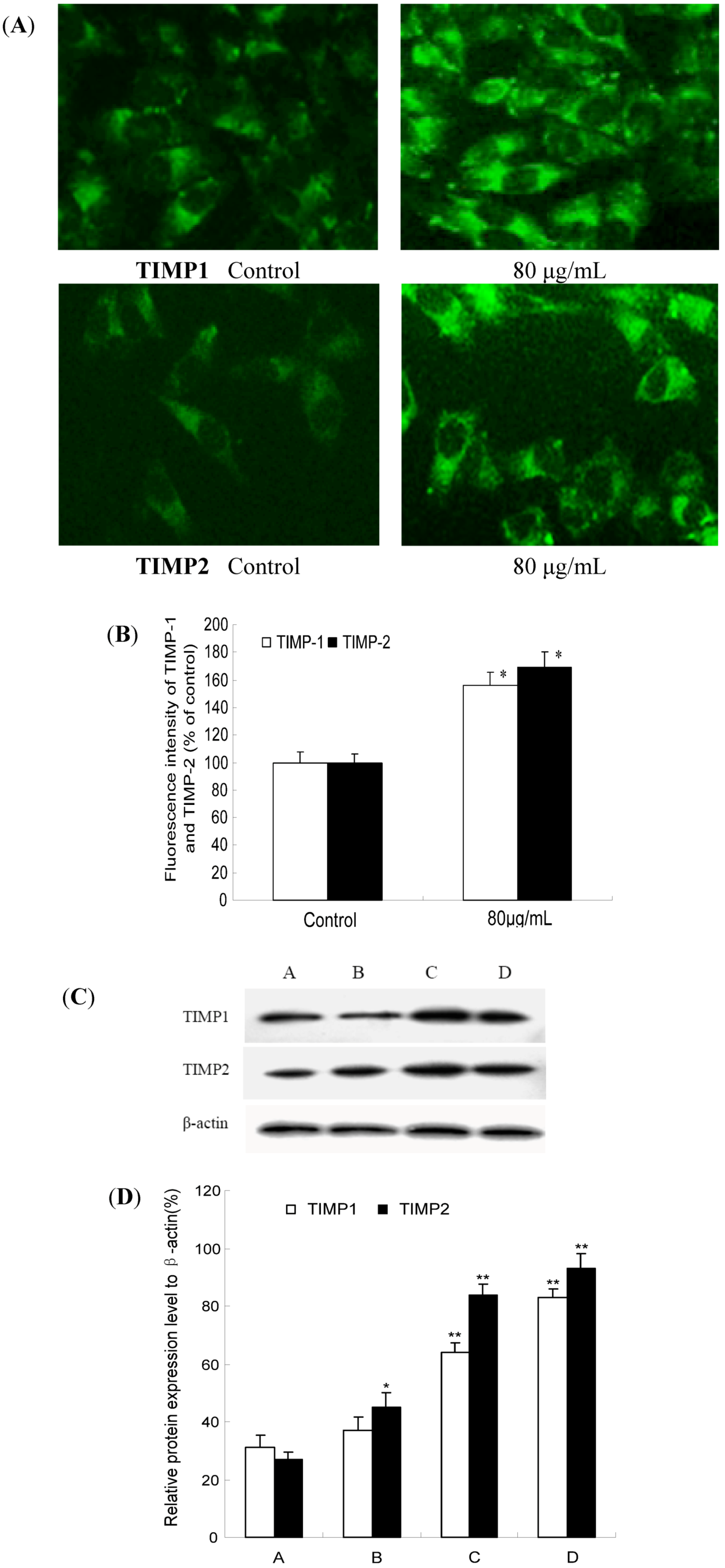
2.6. TF-SB Increases TIMP-1 and TIMP-2 Protein Expression with Immunofluorescence and Western Blot Analysis
3. Experimental
3.1. Reagents
3.2. Cell Line and Cell Culture
3.3. Preparation of TF-SB from Scutellaria barbata D. Don
3.4. MTT Assay for the Proliferation of MHCC97H Cells
3.5. Wound Healing Assay
3.6. Invasion Assay
3.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.8. Immunofluorescence Staining
3.9. Western Blot Analysis
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the plant are available from the authors.
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Farazi1, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef]
- Vickers, A. Botanical medicines for the treatment of cancer: Rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest. 2002, 20, 1069–1079. [Google Scholar] [CrossRef]
- Wang, Z.D.; Huang, C.; Li, Z.F.; Yang, J.; Li, B.H.; Liang, R.R.; Dai, Z.J.; Liu, Z.W. Chrysanthemum indicum ethanolic extract inhibits invasion of hepatocellular carcinoma via regulation of MMP/TIMP balance as therapeutic target. Oncol. Rep. 2010, 23, 413–421. [Google Scholar]
- Bjorklund, M.; Koivunen, E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta 1755, 37–69. [Google Scholar]
- Khan, S.T.; Pixley, R.A.; Liu, Y.; Bakdash, N.; Gordon, B.; Agelan, A.; Huang, Y.; Achary, M.P.; Colman, R.W. Inhibition of metastasis of syngeneic murine melanoma in vivo and vasculogenesis in vitro by monoclonal antibody C11C1 targeted to domain 5 of high molecular weight kininogen. Cancer Immunol. Immunother. 2010, 59, 1885–1893. [Google Scholar] [CrossRef]
- Kang, J.H.; Han, I.H.; Sung, M.K.; Yoo, H.; Kim, Y.K.; Kim, J.S.; Kawada, T.; Yu, R. Soybean saponin inhibits tumor cell metastasis by modulating expressions of MMP-2, MMP-9 and TIMP-2. Cancer Lett. 2008, 261, 84–92. [Google Scholar] [CrossRef]
- Hu, Y.H.; Yu, L.J.; Shao, E.D.; Wu, J.L.; Ji, J.W. The regulating role of mutant IkappaBalpha in expression of TIMP-2 and MMP-9 in human glioblastoma multiform. Chin. Med. J. (Engl.) 2009, 122, 205–211. [Google Scholar]
- Figueira, R.C.; Gomes, L.R.; Neto, J.S.; Silva, F.C.; Silva, I.D.; Sogayar, M.C. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC. Cancer 2009, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, G.; Bergamini, C.; Marinosci, F.; Fransvea, E.; Quaranta, M.; Lupo, L.; Schiraldi, O.; Antonaci, S. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int. J. Cancer 2002, 97, 425–431. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, D.K.; Kim, D.I.; Lee, Y.C.; Chang, Y.C.; Kim, C.H. Inhibitory effects of Scutellaria barbata D. Don on human uterine leiomyomal smooth muscle cell proliferation through cell cycle analysis. Int. Immunopharmacol. 2004, 4, 447–454. [Google Scholar] [CrossRef]
- Lin, C.C.; Shieh, D.E. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicaleinand wogonin. Am. J. Chin. Med. 1996, 24, 31–36. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, D.I.; Song, Y.L.; Lee, Y.C.; Kim, H.M.; Kim, C.H. Differential inhibition of Scutellaria barbata D. Don (Lamiaceae) on HCG-promoted proliferation of cultured uterine leiomyomal and myometrial smooth muscle cells. Immunopharmacol. Immunotoxicol. 2004, 26, 329–342. [Google Scholar] [CrossRef]
- Goh, D.; Lee, Y.H.; Ong, E.S. Inhibitory effects of a chemically standardized extract from Scutellaria barbata in human colon cancer cell lines, LoVo. J. Agric. Food Chem. 2005, 53, 8197–8204. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, J.; Jie, C.; Xing, D.; Zhang, Y. Anticanceractivity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004, 75, 2233–2244. [Google Scholar] [CrossRef]
- Cha, Y.Y.; Lee, E.O.; Lee, H.J.; Park, Y.D.; Ko, S.G.; Kim, D.H.; Kim, H.M.; Kang, I.C.; Kim, S.H. Methylene chloride fraction of Scutellaria barbata induces apoptosis in human U937 leukemia cells via the mitochondrial signaling pathway. Clin. Chim. Acta 2004, 348, 41–48. [Google Scholar] [CrossRef]
- Suh, S.J.; Yoon, J.W.; Lee, T.K.; Jin, U.H.; Kim, S.L.; Kim, M.S.; Kwon, D.Y.; Lee, Y.C.; Kim, C.H. Chemoprevention of Scutellaria bardata on Human Cancer Cells and Tumorigenesis in Skin Cancer. Phytother Res. 2007, 21, 135–141. [Google Scholar] [CrossRef]
- Dai, Z.J.; Wang, X.J.; Li, Z.F.; Ji, Z.Z.; Ren, H.T.; Tang, W.; Liu, X.X.; Kang, H.F.; Guan, H.T.; Song, L.Q. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World J. Gastroenterol. 2008, 14, 7321–7328. [Google Scholar]
- Lee, T.K.; Cho, H.L.; Kim, D.I.; Lee, Y.C.; Kim, C.H. Scutellaria barbata D. Don induces c-fos gene expression in human uterine leiomyomal cells by activating beta2-adrenergic receptors. Int. J. Gynecol. Cancer 2004, 14, 526–531. [Google Scholar] [CrossRef]
- Dai, S.J.; Sun, J.Y.; Ren, Y.; Liu, K.; Shen, L. Bioactive ent-clerodane diterpenoids from Scutellaria barbata. Planta Med. 2007, 73, 1217–1220. [Google Scholar] [CrossRef]
- Qu, G.W.; Yue, X.D.; Li, G.S.; Yu, Q.Y.; Dai, S.J. Two new cytotoxic ent-clerodane diterpenoids from Scutellaria barbata. J. Asian Nat. Prod. Res. 2010, 12, 859–864. [Google Scholar] [CrossRef]
- Dai, S.J.; Peng, W.B.; Shen, L.; Zhang, D.W.; Ren, Y. New norditerpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 2011, 15, 1–6. [Google Scholar]
- Mi, X.; Zhu, R. Simultaneous determination of 7 active ingredients in Scutellaria barbata D. Don by capillary micellar electrokinetic chromatography. Se Pu 2010, 28, 209–214. [Google Scholar]
- Dai, Z.J.; Gao, J.; Li, Z.F.; Ji, Z.Z.; Kang, H.F.; Guan, H.T.; Diao, Y.; Wang, B.F.; Wang, X.J. In vitro and in vivo antitumor activity of Scutellaria barbate extract on murine liver cancer. Molecules 2011, 16, 4389–4400. [Google Scholar] [CrossRef]
- Klawitter, J.; Klawitter, J.; Gurshtein, J.; Corby, K.; Fong, S.; Tagliaferri, M.; Quattrochi, L.; Cohen, I.; Shtivelman, E.; Christians, U. Bezielle (BZL101)-induced oxidative stress damage followed by redistribution of metabolic fluxes in breast cancer cells: A combined proteomic and metabolomic study. Int. J. Cancer 2011, 129, 2945–2957. [Google Scholar] [CrossRef]
- Marconett, C.N.; Morgenstern, T.J.; San-Roman, A.K.; Sundar, S.N.; Singhal, A.K.; Firestone, G.L. BZL101, a phytochemical extract from the Scutellaria barbata plant, disrupts proliferation of human breast and prostate cancer cells through distinct mechanisms dependent on the cancer cell phenotype. Cancer Biol. Ther. 2010, 10, 397–405. [Google Scholar] [CrossRef]
- Ji, X.N.; Ye, S.L.; Li, Y.; Tian, B.; Chen, J.; Gao, D.M.; Chen, J.; Bao, W.H.; Liu, Y.K.; Tang, Z.Y. Contributions of lung tissue extracts to invasion and migration of human hepatocellular carcinoma cells with various metastatic potentials. J. Cancer Res. Clin. Oncol. 2003, 129, 556–564. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Fan, J.; Tan, C.J.; Qiu, S.J.; Huang, X.W.; Tang, Z.Y. Sirolimus inhibits the growth and metastatic progression of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 715–722. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, L.; Huang, Z.L.; Zheng, Q.; Li, Q.S.; Tang, Z.Y. Herbal extract “Songyou Yin” inhibits tumor growth and prolongs survival in nude mice bearing human hepatocellular carcinoma xenograft with high metastatic potential. J. Cancer Res. Clin. Oncol. 2009, 135, 1245–1255. [Google Scholar] [CrossRef]
- Rugo, H.; Shtivelman, E.; Perez, A.; Vogel, C.; Franco, S.; Tan, C.E.; Melisko, M.; Tagliaferri, M.; Cohen, I.; Shoemaker, M.; et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res. Treat. 2007, 105, 17–28. [Google Scholar] [CrossRef]
- Perez, A.T.; Arun, B.; Tripathy, D.; Tagliaferri, M.A.; Shaw, H.S.; Kimmick, G.G.; Cohen, I.; Shtivelman, E.; Caygill, K.A.; Grady, D.; et al. A phase IB dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat. 2010, 120, 111–118. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.H.; Kim, J.B.; Nam, S.J.; Yang, J.H.; Kim, J.H.; Lee, J.E. Berberine suppresses TNF-α-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 2008, 13, 2975–2985. [Google Scholar] [CrossRef]
- Han, X.; Yan, D.M.; Zhao, X.F.; Hiroshi, M.; Ding, W.G.; Li, P.; Jiang, S.; Du, B.R.; Du, P.G.; Zhu, X. GHGKHKNK octapeptide (P-5m) inhibits metastasis of HCCLM3 cell lines via regulation of MMP-2 expression in in vitro and in vivo studies. Molecules 2012, 17, 1357–1372. [Google Scholar] [CrossRef]
- Dai, Z.J.; Gao, J.; Ma, X.B.; Yan, K.; Liu, X.X.; Kang, H.F.; Ji, Z.Z.; Guan, H.T.; Wang, X.J. Up-regulation of hypoxia inducible factor-1α by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J. Exp. Clin. Cancer Res. 2012, 31, 28. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, J.Y.; Choi, J.H.; Kim, H.G.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem. Toxicol. 2006, 44, 1890–1896. [Google Scholar] [CrossRef]
- Guan, H.T.; Xue, X.H.; Dai, Z.J.; Wang, X.J.; Li, A.; Qin, Z.Y. Downregulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J. Gastroenterol. 2006, 12, 2901–2907. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dai, Z.-J.; Wang, B.-F.; Lu, W.-F.; Wang, Z.-D.; Ma, X.-B.; Min, W.-L.; Kang, H.-F.; Wang, X.-J.; Wu, W.-Y. Total Flavonoids of Scutellaria barbata Inhibit Invasion of Hepatocarcinoma via MMP/TIMP in Vitro. Molecules 2013, 18, 934-950. https://doi.org/10.3390/molecules18010934
Dai Z-J, Wang B-F, Lu W-F, Wang Z-D, Ma X-B, Min W-L, Kang H-F, Wang X-J, Wu W-Y. Total Flavonoids of Scutellaria barbata Inhibit Invasion of Hepatocarcinoma via MMP/TIMP in Vitro. Molecules. 2013; 18(1):934-950. https://doi.org/10.3390/molecules18010934
Chicago/Turabian StyleDai, Zhi-Jun, Bao-Feng Wang, Wang-Feng Lu, Zhi-Dong Wang, Xiao-Bin Ma, Wei-Li Min, Hua-Feng Kang, Xi-Jing Wang, and Wen-Ying Wu. 2013. "Total Flavonoids of Scutellaria barbata Inhibit Invasion of Hepatocarcinoma via MMP/TIMP in Vitro" Molecules 18, no. 1: 934-950. https://doi.org/10.3390/molecules18010934



