Conductance Studies on Complex Formation between c-Methylcalix[4]resorcinarene and Titanium (III) in Acetonitrile-H2O Binary Solutions
1. Introduction

2. Results and Discussion


| Medium | Log Kf ± SD a | ||
|---|---|---|---|
| 15 °C | 25 °C | 35 °C | |
| Pure AN | 3.59 ± 0.06 | 3.62 ± 0.08 | 3.65 ± 0.20 |
| 57.95% AN-42.05% H2O | 3.29 ± 0.03 | 3.30 ± 0.04 | 3.37 ± 0.18 |
| 34.09% AN-65.91% H2O | 2.86 ± 0.05 | 3.04 ± 0.07 | 3.06 ± 0.07 |
| 18.68% AN-81.32% H2O | 2.97 ± 0.13 | 2.86 ± 0.30 | 3.02 ± 0.10 |
| 7.93% AN-92.07% H2O | 2.62 ± 0.16 | 2.83 ± 0.17 | 2.87 ± 0.21 |
| Pure H2O | c | c | c |

 and
and  of the complexation reactions in different AN–H2O mixtures were evaluated from the temperature dependence of the formation constants by applying a linear least-squares analysis according to the Van’t Hoff equation. The Van’t Hoff plots of ln Kf vs. 1/T are shown in Figure 5. The enthalpies and entropies of complexation were determined in the usual manner from the slopes and intercepts of the plots, respectively, and the results are listed in Table 2.
of the complexation reactions in different AN–H2O mixtures were evaluated from the temperature dependence of the formation constants by applying a linear least-squares analysis according to the Van’t Hoff equation. The Van’t Hoff plots of ln Kf vs. 1/T are shown in Figure 5. The enthalpies and entropies of complexation were determined in the usual manner from the slopes and intercepts of the plots, respectively, and the results are listed in Table 2.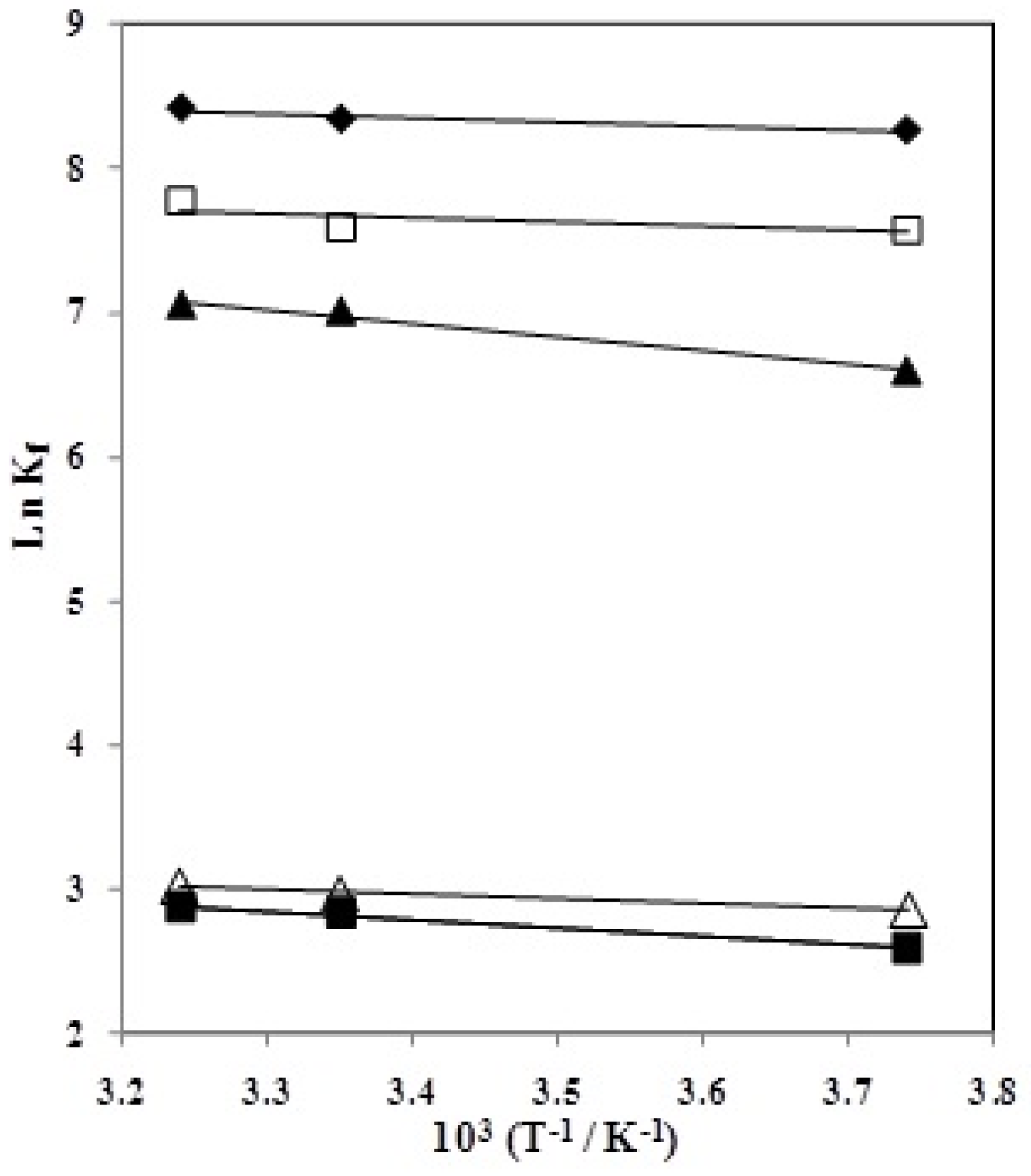
| Medium | 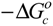 ± SDa kJ/mol ± SDa kJ/mol |  ± SDa kJ/mol ± SDa kJ/mol |  ± SDa J/mol.⁰K ± SDa J/mol.⁰K |
|---|---|---|---|
| Pure AN | 20.66 ± 0.47 | 5.54 ± 0.12 | 87.90 ± 1.52 |
| 57.95% AN-42.05% H2O | 18.82 ± 0.23 | 7.20 ± 3.41 | 87.27 ± 11.41 |
| 34.09% AN-65.91% H2O | 17.37 ± 0.41 | 16.66 ± 7.25 | 114.18 ± 24.33 |
| 18.68% AN-81.32% H2O | 16.95 ± 0.73 | 13.90 ± 2.36 | 103.47 ± 7.52 |
| 7.93% AN-92.07% H2O | 16.18 ± 0.97 | 23.80 ± 9.05 | 134.10 ± 30.19 |
 ) at 25 °C for CMCR-Ti(OH)(OH2)52+ complexes in pure AN and AN–H2O binary systems are listed in Table 2. The given experimental values of standard enthalpy (
) at 25 °C for CMCR-Ti(OH)(OH2)52+ complexes in pure AN and AN–H2O binary systems are listed in Table 2. The given experimental values of standard enthalpy (  ) and standard entropy (
) and standard entropy (  ) show that, in most cases, the complexes are enthalpy destabilized, but entropy stabilized. Therefore, the entropy of the complexation reactions is the principal driving force for the formation of these complexes in most solvent systems. The experimental results show that, in most cases, the change in standard enthalpy for the complexation reaction between ligand and ion is negligible; therefore, it seems that the complexation processes in most of the solvent systems are probably athermic.
) show that, in most cases, the complexes are enthalpy destabilized, but entropy stabilized. Therefore, the entropy of the complexation reactions is the principal driving force for the formation of these complexes in most solvent systems. The experimental results show that, in most cases, the change in standard enthalpy for the complexation reaction between ligand and ion is negligible; therefore, it seems that the complexation processes in most of the solvent systems are probably athermic.3. Experimental
3.1. Materials
3.2. Methodology
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Brown, P.O.; Enright, G.D.; Ripmeester, J.A. Cationic guests in extended anionic c-methylcalix [4] resorcinarene-inorganic frameworks: Exercising conformational control over c-methylcalix [4] resorcinarene. J. Supramol. Chem. 2002, 2, 497–500. [Google Scholar]
- Lepakshaiah, M.; Guru Row, T.N. Solid state stacked trimers of 1, 10-phenanthroline in a supramolecular tubular motif of C-methylcalix [4] resorcinarene. J. Mol. Struct. 2009, 918, 10–13. [Google Scholar]
- Ma, B.Q.; Zhang, Y.; Coppens, P. Multiple structures in supramolecular solids: Benzophenone embedded in three different C-Methylcalix [4] resorcinarene/bipyridine frameworks. Cryst. Growth Des. 2001, 1, 271–275. [Google Scholar]
- Shivanyuk, A.; Saadioui, M.; Broda, F.; Thondorf, I.; Vysotsky, M.O.; Rissanen, K.; Kolehmainen, E.; Böhmer, V. Sterically and Guestâ Controlled Self Assembly of Calix [4] arene Derivatives. Chem.-Eur. J. 2004, 10, 2138–2148. [Google Scholar] [CrossRef]
- Memon, S.; Akceylan, E.; Sap, B.; Tabakci, M.; Roundhill, D.M.; Yilmaz, M. Polymer supported calix [4] arene derivatives for the extraction of metals and dichromate anions. J. Polym. Environ. 2003, 11, 67–74. [Google Scholar]
- Zhang, Y.; Kim, C.D.; Coppens, P. Does C-methylcalix [4] resorcinarene always adopt the crown shape conformation? A resorcinarene/bipyridine/decamethylruthenocene supramolecular clathrate with a novel framework structure. Chem. Commun. 2000, 23, 2299–2300. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Lukashenko, S.S.; Kosacheva, E.M.; Rizvanova, L.Z.; Gainanova, G.A.; Knyazeva, I.R.; Burilov, A.R.; Kudryavtseva, L.A.; Konovalov, A.I. Supramolecular systems based on poly (ethyleneimines) and calix [4] resorcinarenes with alkylphosphonate fragments. Aggregation and catalytic activity. Russ. Chem. Bull. 2007, 56, 959–966. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, R.; Kaur, I.; Sharma, V.; Kumar, M. Mercury (II) ion-selective electrodes based on p-tert-butyl calix [4] crowns with imine units. Anal. Sci. 2004, 20, 811–814. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Barnes, C.E. Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J. Chem. Soc. Dalton Trans. 1999, 8, 1201–1202. [Google Scholar]
- Ding, X.; Nomura, M.; Suzuki, T.; Sugiyama, Y.; Kaneshiki, T.; Fujii, Y. Chromatographic zinc isotope separation by phenol formaldehyde benzo crown resin. J. Chromatogr. A 2006, 1113, 182–185. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, S.; Chandra, S. Chemical sensor for lanthanum (III) determination using aza-crown as ionophore in poly (vinyl chloride) matrix. Anal. Chim. Acta 2003, 486, 199–207. [Google Scholar] [CrossRef]
- Kuhn, R.; Riester, D.; Fleckenstein, B.; Wiesmüller, K.H. Evaluation of an optically active crown ether for the chiral separation of di-and tripeptides. J. Chromatogr. A 1995, 716, 371–379. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H. Crown ethers for chemical analysis: A Review. Anal. Lett. 1982, 15, 1197–1276. [Google Scholar] [CrossRef]
- Rezayi, M.; Ahmadzadeh, S.; Kassim, A.; Lee, Y.H. Thermodynamic studies of complex formation between Co (Salen) ionophore with chromate (II) ions in AN-H2O binary solutions by the conductometric method. Int. J. Electrochem. Sci. 2011, 6, 6350–6359. [Google Scholar]
- Ahmadzadeh, S.; Kassim, A.; Rezayi, M.; Rounaghi, G.H. Thermodynamic Study of the Complexation of p-Isopropylcalix [6] arene with Cs+ Cation in Dimethylsulfoxide-Acetonitrile Binary Media. Molecules 2011, 16, 8130–8142. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Kassim, A.; Rezayi, M.; Abdollahi, Y.; Hossein, G. A conductometric study of complexation reaction between meso-octamethylcalix [4] pyrrole with titanium cation in acetonitrile-ethanol binary mixtures. Int. J. Electrochem. Sci. 2011, 6, 4749–4759. [Google Scholar]
- Rezayi, M.; Heng, L.Y.; Kassim, A.; Ahmadzadeh, S.; Abdollahi, Y.; Jahangirian, H. Immobilization of Ionophore and Surface Characterization Studies of the Titanium (III) Ion in a PVC-Membrane Sensor. Sensors 2012, 12, 8806–8814. [Google Scholar] [CrossRef]
- Rezayi, M.; Heng, L.Y.; Kassim, A.; Ahmadzadeh, S.; Abdollahi, Y.; Jahangirian, H. Immobilization of tris (2 pyridyl) methylamine in a PVC-Membrane Sensor and Characterization of the Membrane Properties. Chem. Central J. 2012, 6, 40. [Google Scholar] [CrossRef]
- Tavakoly Sany, S.B.; Salleh, A.; Sulaiman, A.H.; Sasekumar, A.; Rezayi, M.; Tehrani, G.M. Heavy metal contamination in water and sediment of the Port Klang coastal area, Selangor, Malaysia. Environ. Earth Sci. 2012, 1–13. [Google Scholar]
- Rezayi, M.; Kassim, A.; Ahmadzadeh, S.; Naji, A.; Ahangar, H.A. Conductometric determination of formation constants of tris (2-pyridyl) methylamine and titanium (III) in water-acetonitryl mixture. Int. J. Electrochem. Sci. 2011, 6, 4378–4387. [Google Scholar]
- Rounaghi, G.H.; Mohajeri, M.; Ahmadzadeh, S.; Tarahomi, S. A thermodynamic study of interaction of Na+ cation with benzo-15-crown-5 in binary mixed non-aqueous solvents. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 365–372. [Google Scholar] [CrossRef]
- Cassaignon, S.; Koelsch, M.; Jolivet, J.P. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 2007, 68, 695–700. [Google Scholar] [CrossRef]
- Sample Availability: Contact the first author.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rezayi, M.; Alias, Y.; Abdi, M.M.; Saeedfar, K.; Saadati, N. Conductance Studies on Complex Formation between c-Methylcalix[4]resorcinarene and Titanium (III) in Acetonitrile-H2O Binary Solutions. Molecules 2013, 18, 12041-12050. https://doi.org/10.3390/molecules181012041
Rezayi M, Alias Y, Abdi MM, Saeedfar K, Saadati N. Conductance Studies on Complex Formation between c-Methylcalix[4]resorcinarene and Titanium (III) in Acetonitrile-H2O Binary Solutions. Molecules. 2013; 18(10):12041-12050. https://doi.org/10.3390/molecules181012041
Chicago/Turabian StyleRezayi, Majid, Yatimah Alias, Mahnaz M. Abdi, Kasra Saeedfar, and Naghmeh Saadati. 2013. "Conductance Studies on Complex Formation between c-Methylcalix[4]resorcinarene and Titanium (III) in Acetonitrile-H2O Binary Solutions" Molecules 18, no. 10: 12041-12050. https://doi.org/10.3390/molecules181012041