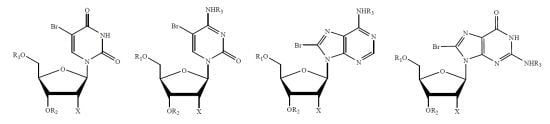
An Efficient and Facile Methodology for Bromination of Pyrimidine and Purine Nucleosides with Sodium Monobromoisocyanurate (SMBI)
Abstract
:1. Introduction
2. Results and Discussion
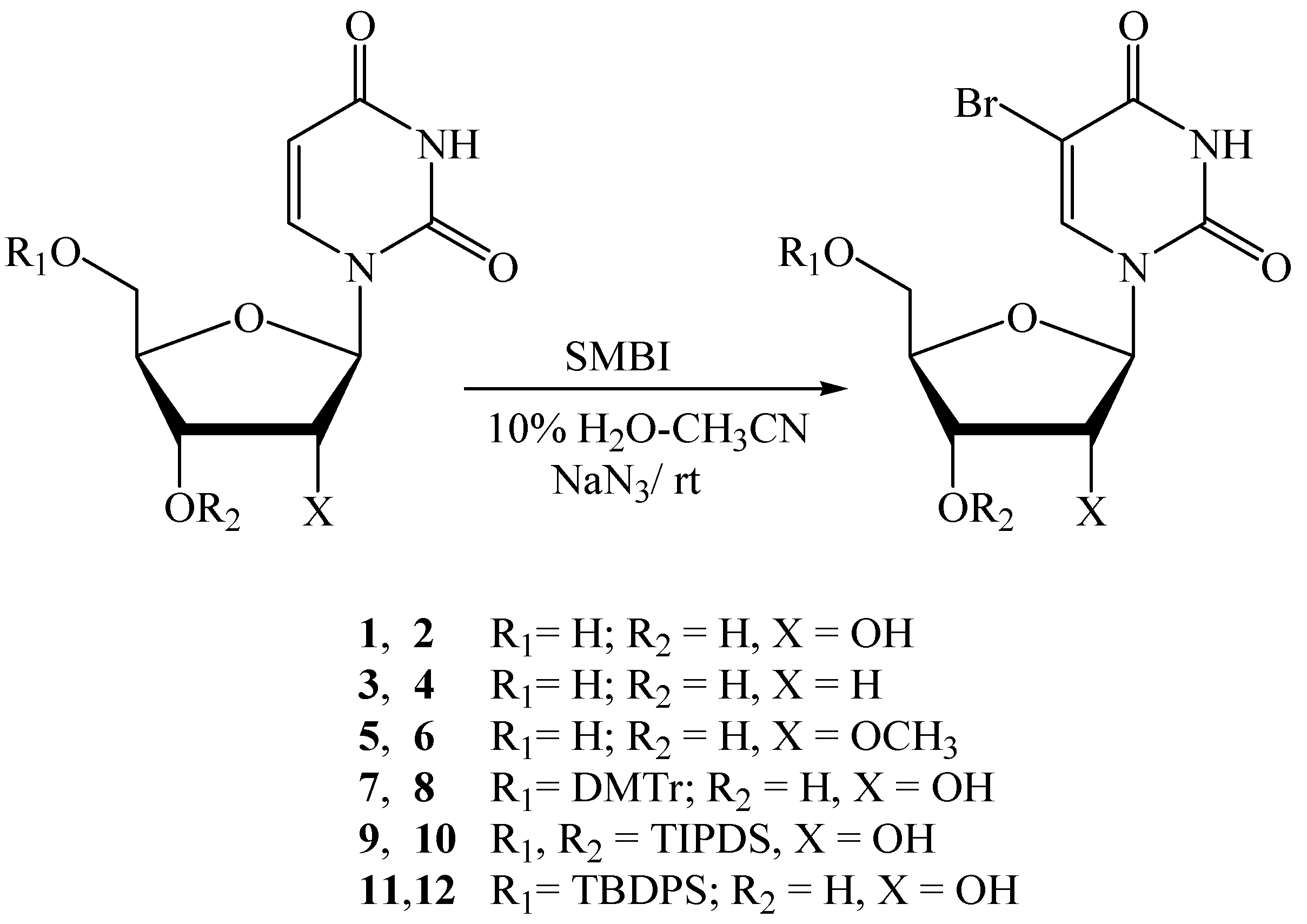
| Entry | Substrate | Product | SMBI (eq.) | NaN3 (eq.) | Solvent | Reaction time (h) | Yield (%) (NMR/TLC) | Isolated yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 1.05 | 4.0 | 10% H2O-CH3CN | 0.5 | 94 a | 79 b |
| 2 | 1 | 2 | 1.05 | 2.0 | 10% H2O-CH3CN | 1.5 | 88 a | - |
| 3 | 1 | 2 | 1.15 | 0 | 10% H2O-CH3CN | 18.0 | 40 c | - |
| 4 | 1 | 2 | 1.15 | 0 | 20% H2O-DMF | 18.0 | 15 c | - |
| 5 | 3 | 4 | 1.05 | 4.0 | 10% H2O-CH3CN | 1.2 | 90 a | 86 d |
| 6 | 5 | 6 | 1.15 | 4.0 | 10% H2O-CH3CN | 1.5 | - | 93 d |
| 7 | 7 | 8 | 1.1 | 4.0 | 10% H2O-CH3CN | 1.0 | 86 a | 81 d |
| 8 | 9 | 10 | 1.1 | 4.0 | 10% H2O-CH3CN | 1.5 | 94 a | 91 d |
| 9 | 11 | 12 | 1.1 | 4.0 | 10% H2O-CH3CN | 1.2 | - | 92 d |
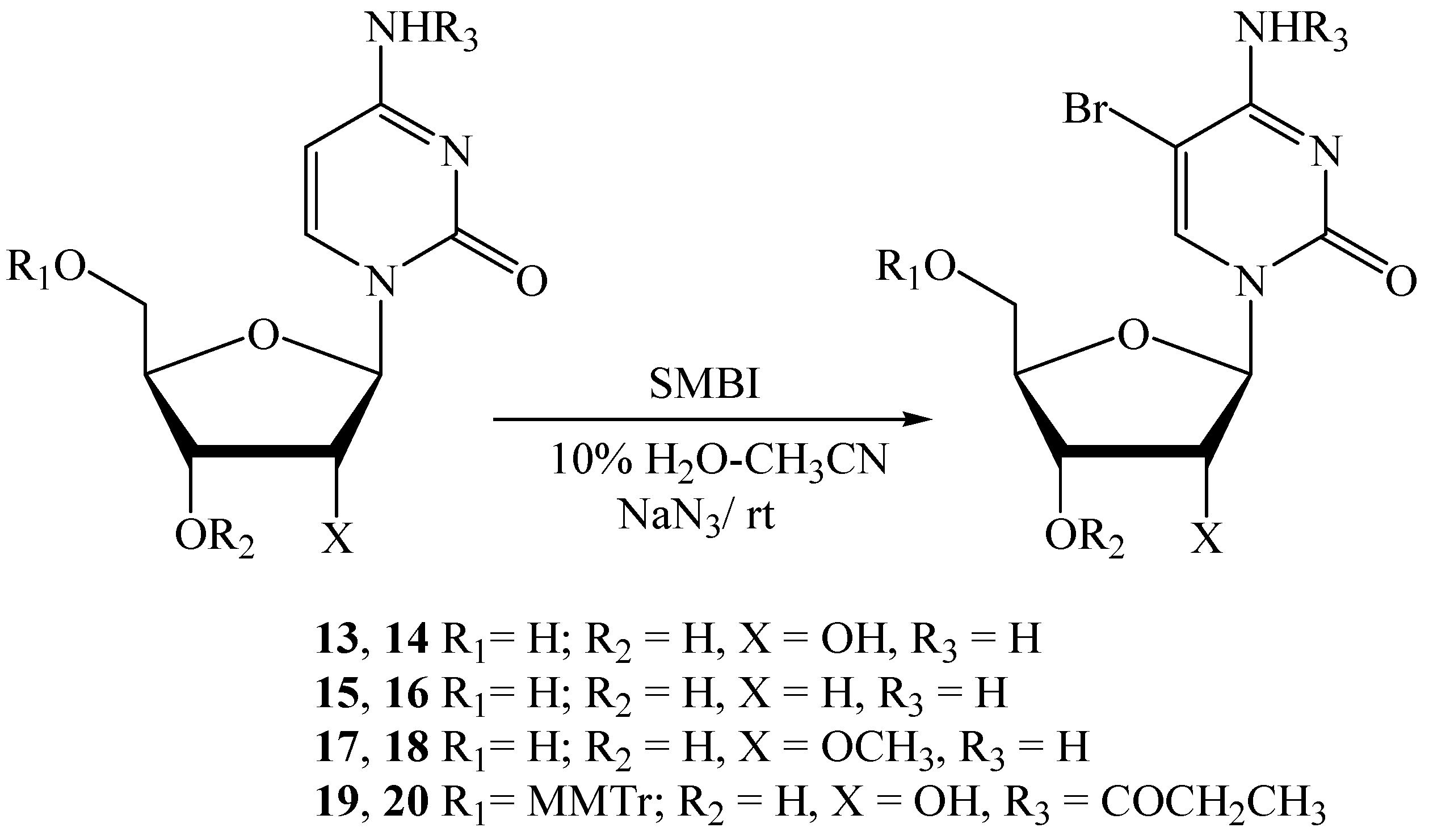
| Entry | Substrate | Product | SMBI (eq.) | NaN3 (eq.) | Solvent | Reaction time (h) | Yield (%) (NMR) | Isolated yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 13 | 14 | 1.45 | 4.0 | 10% H2O-CH3CN | 3.0 | 83 a | 69 b |
| 2 | 13 | 14 | 1.8 | 0 | 20% H2O-DMF | 20.0 | 73 a | - |
| 3 | 15 | 16 | 1.6 | 4.0 | 10% H2O-CH3CN | 3.5 | 73 a | 66 c |
| 4 | 17 | 18 | 1.25 | 4.0 | 10% H2O-CH3CN | 2.0 | - | 78 c |
| 5 | 19 | 20 | 1.25 | 4.0 | 10% H2O-CH3CN | 2.5 | - | 59 c |
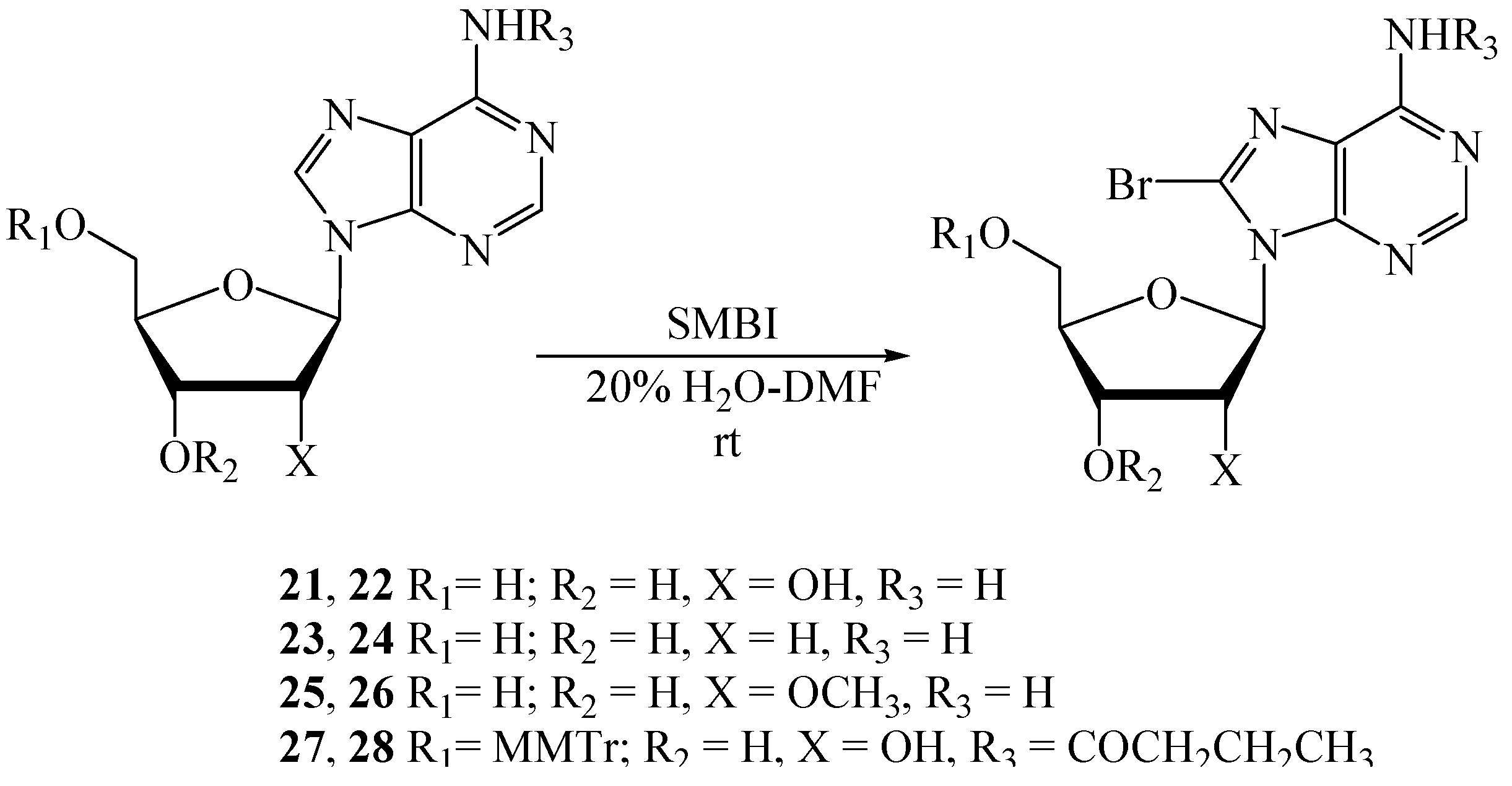
| Entry | Substrate | Product | SMBI (eq.) | Solvent | Reaction time (h) | Yield (%) (NMR/TLC) | Isolated yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 21 | 22 | 1.4 | 20% H2O-DMF | 2.5 | 89 a | 73 b |
| 2 | 23 | 24 | 1.3 | 20% H2O-DMF | 2.0 | 93 a | 88 d |
| 3 | 25 | 26 | 1.45 | 20% H2O-DMF | 2.0 | 78 a | 71 d |
| 4 | 27 | 28 | 1.2 | 20% H2O-DMF | 4.0 | <5 c | - |
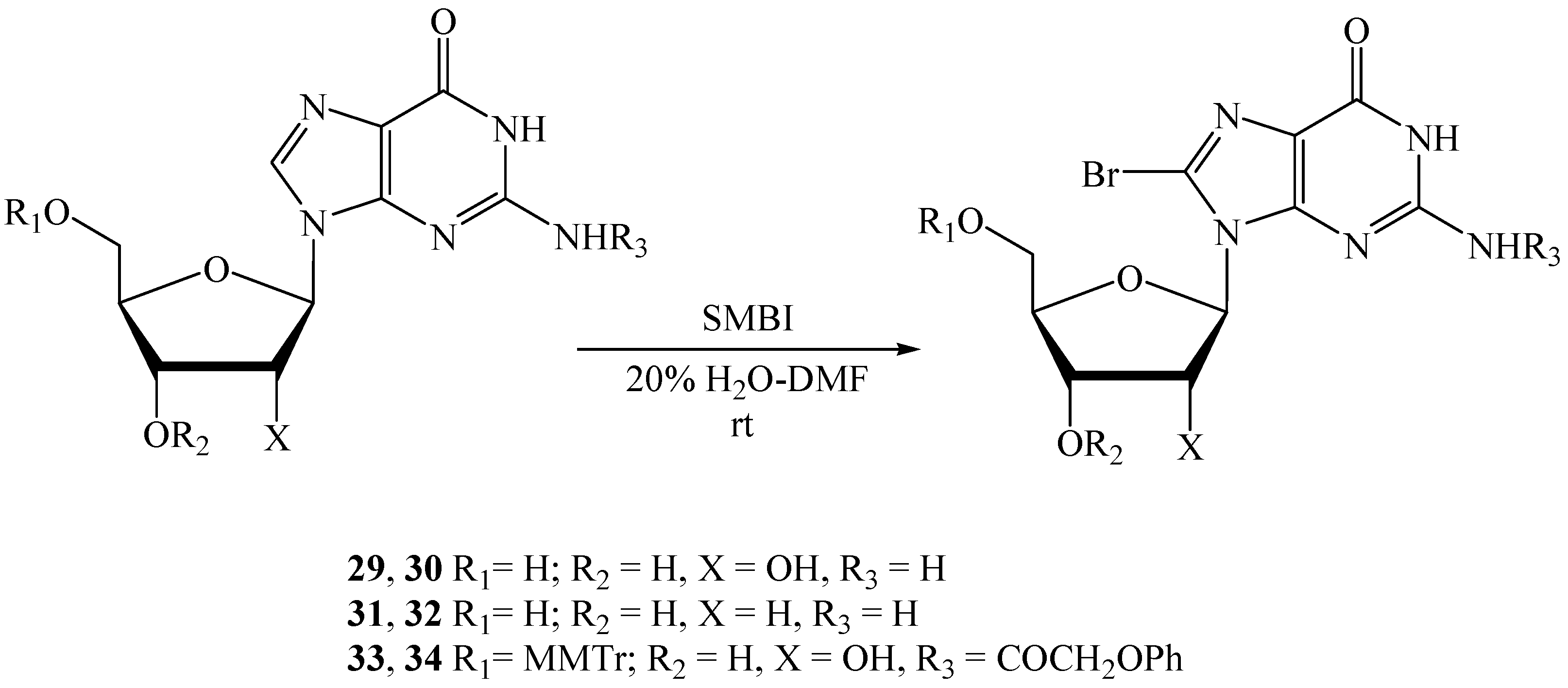
| 5 | 29 | 30 | 1.5 | 20% H2O-DMF | 4.0 | 15 c | - |
| 6 | 31 | 32 | 1.5 | 20% H2O-DMF | 4.0 | 62 a | 57 d |
| 7 | 33 | 34 | 1.25 | 20% H2O-DMF | 2.0 | - | 96 d |
3. Experimental
3.1. General
3.2. Typical Procedure for the Bromination of Pyrimidine Nucleosides and Their Derivatives
3.3. Typical Procedure for Bromination of Purine Nucleosides and Their Derivatives
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Van Aershot, A.; Everaert, D.; Balzarini, J.; Augustyns, K.; Jie, L.; Janssen, G.; Peeters, O.; Blaton, N.; DeRanter, C.; DeClercq, E.; Herdewijn, P. Synthesis and anti-HIV evaluation of 2',3'-dideoxyribo-5-chloropyrimidine analogues: Reduced toxicity of 5-chlorinated 2',3'-dideoxynucleoside. J. Med. Chem. 1990, 33, 1833–1839. [Google Scholar] [CrossRef]
- Fox, L.; Dorbersen, M.J.; Greer, S. Incorporation of 5-substituted analogs of deoxycytidine into DNA of herpes simplex virus-infected or -transformed cells without deamination to the thymidine analog. Antimicrob. Agents Chemother. 1983, 23, 465–476. [Google Scholar] [CrossRef]
- Harki, D.A.; Graci, J.D.; Galarraga, J.E.; Chain, W.J. Synthesis and antiviral activity of 5-substituted cytidine analogues: Identification of a potent inhibitor of viral RNA-dependent RNA polymerases. J. Med. Chem. 2006, 49, 6166–6169. [Google Scholar] [CrossRef]
- Perigaud, C.; Gosselin, G.; Imbach, J.L. Nucleoside analogues as chemotherapeutic agents: A review. Nucleosides Nucleotides 1992, 11, 903–945. [Google Scholar] [CrossRef]
- Clercq, E.; Balzarini, J.; Torrence, P.F.; Mertes, M.P.; Schmidt, C.L.; Shugar, D.; Barr, P.J.; Jones, A.S.; Verhelst, G.; Walker, R.T. Thymidylate synthetase as target enzyme for the inhibitory activity of 5-substituted 2′-deoxyuridines on mouse leukemia L1210 cell growth. Mol. Pharmacol. 1981, 19, 321–330. [Google Scholar]
- Szybalski, W. X-ray sensitization by halopyrimidines. Cancer Chemother. Rep. 1974, 58, 539–557. [Google Scholar]
- Mercer, J.R.; Xu, L.H.; Knaus, E.E.; Wiebe, L.I. Synthesis and tumor uptake of 5-82Br- and 5-131I-labeled 5-halo-1-(2-fluoro-2-deoxy-β-d-ribofuranosyl)uracils. J. Med. Chem. 1989, 32, 1289–1294. [Google Scholar] [CrossRef]
- Agrofoglio, L.A.; Gillaizeau, I.; Saito, Y. Palladium-assisted routes to nucleosides. Chem. Rev. 2003, 103, 1875–1916. [Google Scholar] [CrossRef]
- Amann, N.; Wagenknecht, H.-A. Preparation of pyrenyl-modified nucleosides via Suzuki-Miyaura cross-coupling reactions. Synlett 2002, 5, 687–691. [Google Scholar] [CrossRef]
- Clima, L.; Bannwarth, W. Building-block approach for the straightforward incorporation of a new FRET (Fluorescence-Resonance-Energy Transfer) system into synthetic DNA. Helv. Chim. Acta 2008, 91, 165–175. [Google Scholar] [CrossRef]
- Veliz, E.A.; Beal, P.A. 6-Bromopurine nucleosides as reagents for nucleoside analogue synthesis. J. Org. Chem. 2001, 66, 8592–8598. [Google Scholar] [CrossRef]
- De Napoli, L.; Messere, A.; Montesarchio, D.; Piccialli, G.; Varra, M. 6-Chloroxanthosine, a useful intermediate for the efficient syntheses of [6-15N]-isoguanosine, isoinosine and other purine nucleoside analogues. Nucleosides Nucleotides 1997, 16, 183–191. [Google Scholar] [CrossRef]
- De Napoli, L.; Messere, A.; Montesarchio, D.; Piccialli, G.; Santacroce, C.; Varra, M. Reaction of 3',5'-di-O-acetyl-2'-deoxyinosine with the chlorinating agent PPh3-CCl4: Synthesis of the 6-chloroderivative and of a new base linked dimer, useful intermediate to 15N-1-labelled 2'-deoxxyinosine. J. Chem. Soc. Perkin Trans. 1 1994, 1994, 923–925. [Google Scholar]
- Ueda, T. Synthesis and Reaction of Pyrimidine Nucleosides. In Chemistry of Nucleosides and Nucleotides; Townsend, L.B., Ed.; Plenum Press: New York, NY, USA, 1988; Volume 1, pp. 1–95. [Google Scholar]
- Fukuhara, T.K.; Visser, D.W. Pyrimidine nucleoside antagonisty. J. Biol. Chem. 1951, 190, 95–101. [Google Scholar]
- Duval, J.; Ebel, J.P. Halogenation of nucleic acids: Action of bromine on bases and nucleosides in dimethylformamide medium. Bull. Soc. Chim. Biol. 1964, 46, 1059–1071. [Google Scholar]
- Fukuhara, T.K.; Visser, D.W. Cytidine derivatives. J. Am. Chem. Soc. 1955, 77, 2393–2395. [Google Scholar] [CrossRef]
- Frisch, D.M.; Visser, D.W. 5-Bromodeoxycytidine and 5-chlorodeoxycytidine. J. Am. Chem. Soc. 1959, 81, 1756–1758. [Google Scholar] [CrossRef]
- Srivastava, P.C.; Nagpal, K.L. Bromination of nucleosides. Experientia 1970, 26, 220. [Google Scholar] [CrossRef]
- Kumar, R.; Wiebe, L.I.; Eknaus, E. A mild and efficient methodology for the synthesis of 5-halogeno uracil nucleosides that occurs via a 5-halogeno-6-azido-5,6-dihydro intermediate. Can. J. Chem. 1994, 72, 2005–2010. [Google Scholar] [CrossRef]
- Kumar, V.; Yap, J.; Muroyama, A.; Malhotra, S. Highly efficient method for C-5 halogenation of pyrimidine-based nucleosides in ionic liquid. Synthesis 2009, 23, 3957–3962. [Google Scholar]
- Ryu, E.K.; MacCoss, M. New Procedure for the chlorination of pyrimidine and purine nucleosides. J. Org. Chem. 1981, 46, 2819–2923. [Google Scholar] [CrossRef]
- Asakura, J.; Robins, M.J. Cerium (IV) mediated halogenation at C-5 of uracil derivatives. J. Org. Chem. 1990, 55, 4928–4933. [Google Scholar] [CrossRef]
- Ross, S.A.; Burrows, C.J. Bromination of pyrimidines using bromide and monoperoxysulfate: A competition study between cytidine, uridine and thymidine. Tetrahedron Lett. 1997, 38, 2805–2808. [Google Scholar] [CrossRef]
- Holmes, R.E.; Robins, R.K. Purine nucleosides. VII. Direct bromination of adenosine, deoxyadenosine, guanosine, and related purine nucleosides. J. Am. Chem. Soc. 1964, 86, 1242–1245. [Google Scholar] [CrossRef]
- Ghanty, U.; Fostvedt, E.; Valenzuela, R.; Beal, P.A.; Burrows, C.J. Promiscuous 8-alkoxyadenosine in the guide strand of an SiRNA: Modulation of silencing efficacy and off-pathway protein binding. J. Am. Chem. Soc. 2012, 134, 17643–17652. [Google Scholar]
- Ohkubo, A.; Nishino, Y.; Yokouchi, A.; Ito, Y.; Noma, Y.; Kakishima, Y.; Masaki, Y.; Tsunoda, H.; Seio, K.; Sekine, M. Stable triplex formation using the strong stacking effect of consecutive thionucleoside moieties. Chem. Commun. 2011, 47, 12556–12558. [Google Scholar] [CrossRef]
- Okada, Y.; Yokozawa, M.; Akiba, M.; Oishi, K.; O-kawa, K.; Akeboshi, T.; Kawamura, Y.; Inokuma, S.; Nakamura, Y.; Nishimura, J. Bromination by means of sodium monobromoisocyanurate (SMBI). Org. Biomol. Chem 2003, 1, 2506–2511. [Google Scholar] [CrossRef]
- Sumida, T.; Kikuchi, S.; Imafuku, K. Bromination of azulene derivatives with sodium monobromoisocyanurate. Synthetic Commun. 2004, 34, 4273–4284. [Google Scholar]
- Yokoyama, Y.; Yamaguchi, T.; Sato, M.; Kobayashi, E.; Murakami, Y.; Okuno, H. Chemistry of unprotected amino acids in aqueous solution: Direct bromination of aromatic amino acids with bromoisocyanuric acid sodium salt under strong acidic condition. Chem. Pharm. Bull. 2006, 54, 1715–1719. [Google Scholar] [CrossRef]
- Kumar, J.A.; Srinivasan, N.; Shanmugham, C. Sodium monobromoisocyanurate: A new catalyst for direct synthesis of aryl thiocyanates. Int. J. Chem. 2011, 3, 95–98. [Google Scholar]
- Ebrahimi, M.; Rossi, P.; Rogers, C.; Harbison, G.S. Dependence of 13C-NMR chemical shifts on conformations of RNA nucleosides and nucleotides. J. Magn. Reson. 2001, 150, 1–9. [Google Scholar] [CrossRef]
- Shah, K.; Wu, H.; Rana, T.M. Synthesis of uridine phosphoramidite analogues: Reagents for site-specific incorporation of photoreactive sites into RNA sequence. Bioconjug. Chem. 1994, 5, 508–512. [Google Scholar] [CrossRef]
- Matsuda, A.; Takenuki, K.; Tanaka, M.; Sasaki, T.; Ueda, T. Nucleosides and Nucleotides. 97. Synthesis of new broad spectrum antineoplastic nucleosides, 2'-deoxy-2'-methylidenecytidine (DMDC) and its derivatives. J. Med. Chem. 1991, 34, 812–819. [Google Scholar] [CrossRef]
- Westman, E.; Stawinski, J.; Stromberg, R. RNA synthesis using H-Phosphonates. Synchronizing 2'-OH and N-protection. Collect. Czech. Chem. Commun. 1993, 58, 236–237. [Google Scholar]
- Mamos, P.; Van Aerschot, A.A.; Weyns, N.J.; Herdewijn, P.A. Straightforward C-8 alkylation of adenosine analogues with tetraalkyltin reagents. Tetrahedron Lett. 1992, 33, 2413–2416. [Google Scholar] [CrossRef]
- Wood, S.G.; Upadhya, K.G.; Dalley, N.K.; McKernan, P.A.; Canonico, P.G.; Robins, R.K.; Revankar, G.R. 8-Substituted guanosine and 2'-deoxyguanosine derivatives as potential inducers of the differentiation of friend erythroleukemia cells. J. Med. Chem. 1985, 28, 1194–1198. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 32 and 34 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maity, J.; Stromberg, R. An Efficient and Facile Methodology for Bromination of Pyrimidine and Purine Nucleosides with Sodium Monobromoisocyanurate (SMBI). Molecules 2013, 18, 12740-12750. https://doi.org/10.3390/molecules181012740
Maity J, Stromberg R. An Efficient and Facile Methodology for Bromination of Pyrimidine and Purine Nucleosides with Sodium Monobromoisocyanurate (SMBI). Molecules. 2013; 18(10):12740-12750. https://doi.org/10.3390/molecules181012740
Chicago/Turabian StyleMaity, Jyotirmoy, and Roger Stromberg. 2013. "An Efficient and Facile Methodology for Bromination of Pyrimidine and Purine Nucleosides with Sodium Monobromoisocyanurate (SMBI)" Molecules 18, no. 10: 12740-12750. https://doi.org/10.3390/molecules181012740