Ring-Opening Polymerization of l-Lactic Acid O-Carboxyanhydrides Initiated by Alkoxy Rare Earth Compounds
Abstract
:1. Introduction
2. Results and Discussion
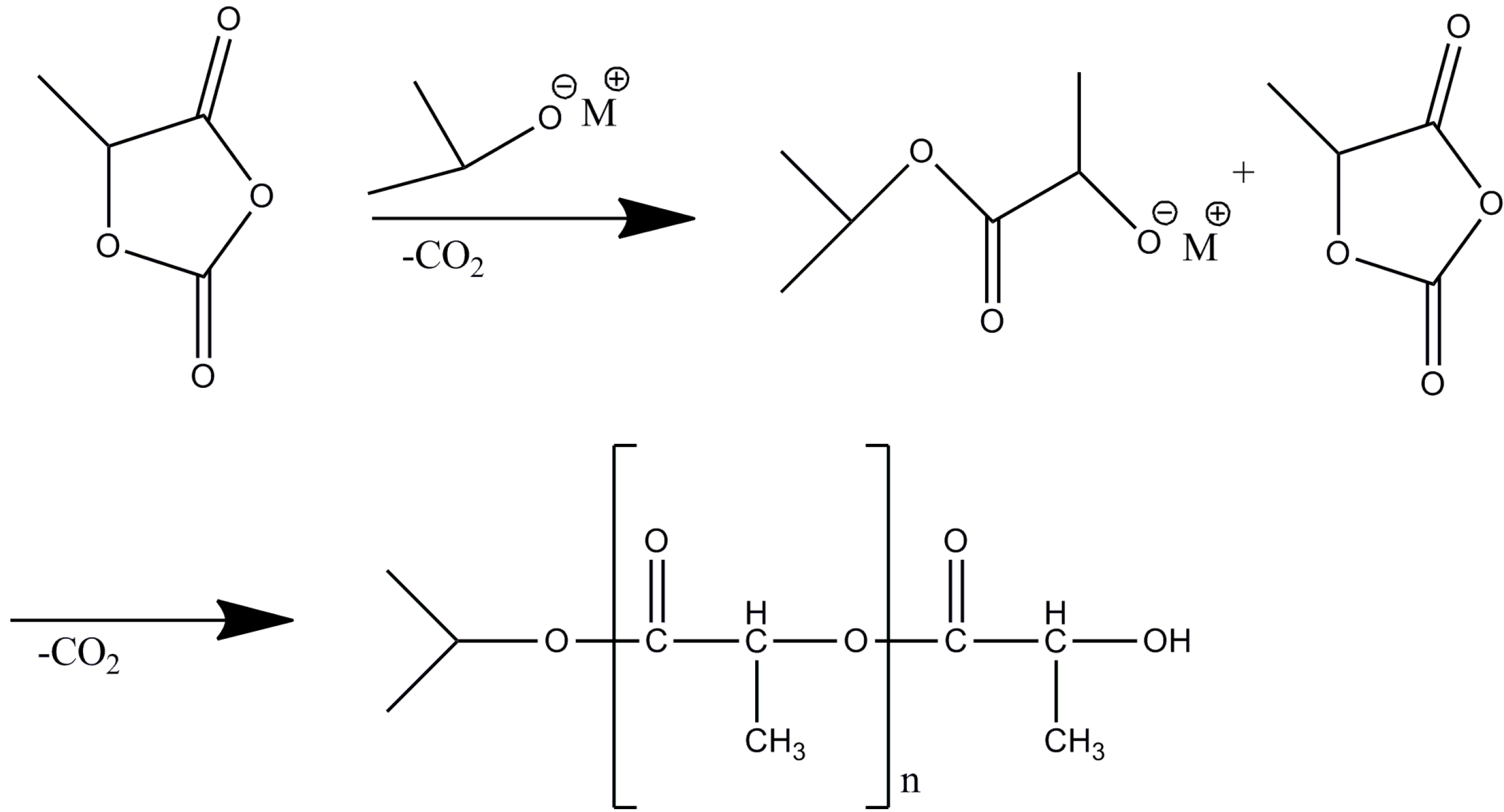
2.1. Polymerization of Lac-OCA
| No. | M/I (molar ratio) | solvent volume (mL) | Mn a (g·moL−1) | Mw/Mn a | Monomer conversion b (%) |
|---|---|---|---|---|---|
| 1 | 27.4:1 | 12.0 | 11,100 | 1.31 | 99.3 |
| 2 | 50:1 | 12.0 | 13,000 | 1.25 | 99.8 |
| 3 | 100:1 | 12.0 | 13,000 | 1.18 | 99.5 |
| 4 | 150:1 | 12.0 | 16,000 | 1.36 | 99.8 |
| 5 | 200:1 | 12.0 | 12,600 | 1.30 | 97.3 |
| 6 | 250:1 | 12.0 | 6,700 | 1.12 | 55.7 |
| 7 | 300:1 | 12.0 | / | / | / |
| 8 | 150:1 | 12.0 | 6,600 | 1.11 | 99.4 |
| No. | Time (h) | Mn a (g·mol−1) | Mw/Mn a | Monomer conversion b (%) |
|---|---|---|---|---|
| 1 | 0.25 | 9200 | 1.18 | 78.36 |
| 2 | 0.5 | 10700 | 1.29 | 90.47 |
| 3 | 0.75 | 10000 | 1.26 | 92.21 |
| 4 | 1.0 | 9700 | 1.32 | 95.32 |
| 5 | 2.0 | 13300 | 1.16 | 98.51 |
| 6 | 4.0 | 12800 | 1.24 | 99.08 |
| 7 | 8.0 | 14200 | 1.15 | 99.17 |
| T (°C) | Mn a (g·mol−1) | Mw/Mn a | Monomer conversion b (%) |
|---|---|---|---|
| 5 | 12,200 | 1.34 | 88.43 |
| 25 | 13,900 | 1.36 | 99.87 |
| 40 | 13,000 | 1.39 | 99.19 |
| 60 | 10,400 | 1.28 | 99.21 |
2.2. NMR Analysis of PLA
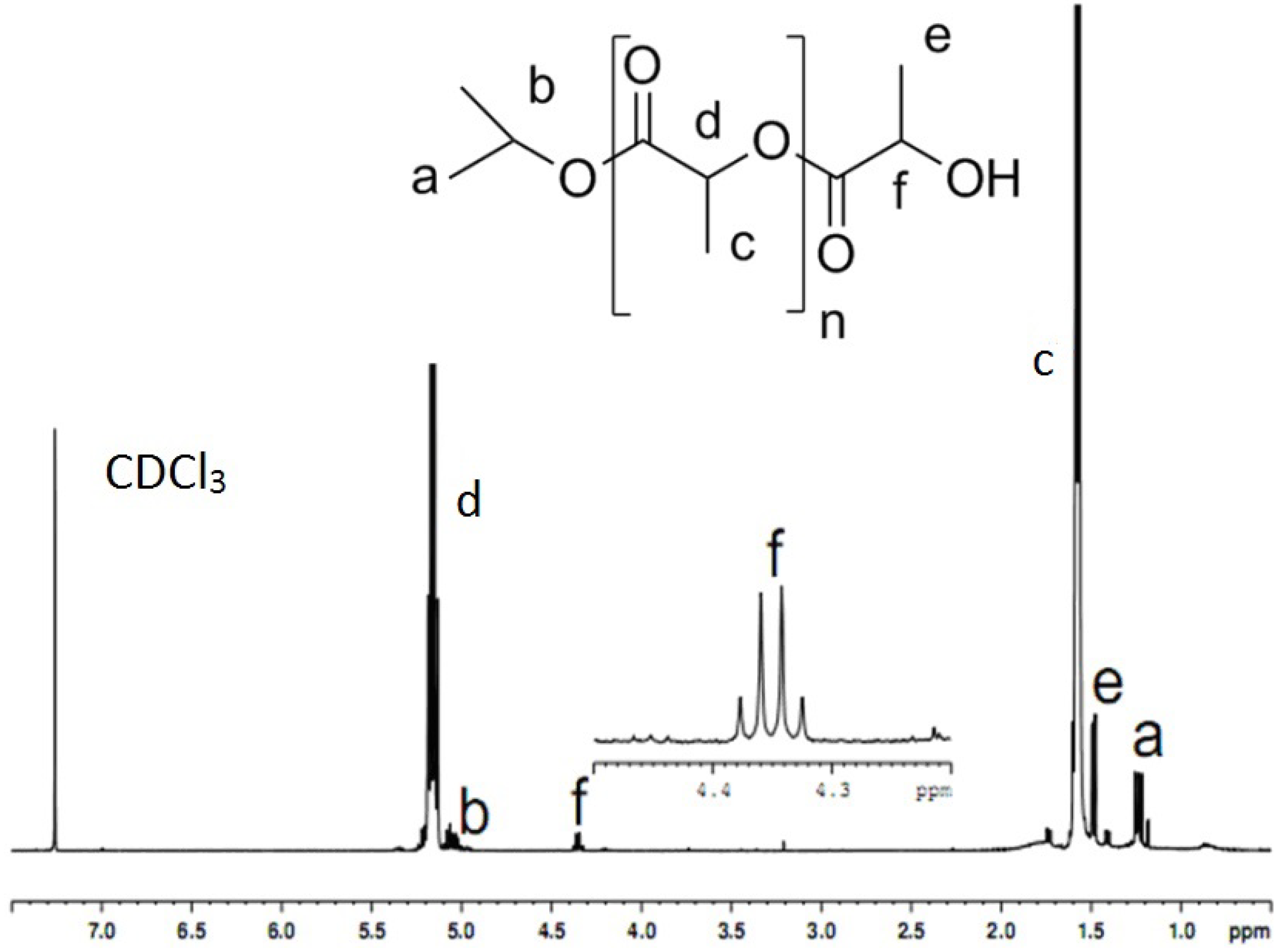
2.3. TGA and DSC analysis of PLAs


3. Experimental
3.1. Materials
3.2. Synthesis of l-lactic Acid O-Carboxyanhydride (Lac-OCA)
3.3. General Procedure for the Polymerization of Lac-OCA
3.4. Characterization Techniques
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Sakellariou, G. Synthesis of Well-Defined Polypeptide-Based Materials via the Ring-Opening Polymerization of α-Amino Acid N-Carboxyanhydrides. Chem. Rev. 2009, 109, 5528–5578. [Google Scholar] [CrossRef]
- Rahul, M.R.; Amol, V.J.; Douglas, E.H. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Jacobsen, S.; Degee, P.H.; Fritz, H.G.; Dubois, P.H.; Jerome, R. Polylactide (PLA)-A New Way of Production. Polym. Eng. Sci. 1999, 39, 1311. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K. Advances in the Properties of Polylactides Based Materials: A Review. J. Biobased Mater. Bio. 2007, 1, 191–209. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Kricheldorf, H.L.; Michael, J. New Polymer Syntheses 8. Synthesis and polymerization of l-Lactic Acid O-Carboxyanhydride (5-MethyI-Dioxolan-2,4-dione). Polym. Bull. 1983, 9, 276–283. [Google Scholar]
- Bonduelle, C.; Martin-Vaca, B.; Bourissou, D. Lipase-Catalyzed Ring-Opening Polymerization of the O-Carboxylic Anhydride Derived from Lactic Acid. Biomacromolecules 2009, 10, 3069–3073. [Google Scholar] [CrossRef]
- Boullay, O.T.; Marchal, E.; Martin-Vaca, B.; Fernando, P.; Bourissou, D. An Activated Equivalent of Lactide toward Organocatalytic Ring-Opening Polymerization. J. Am. Chem. Soc. 2006, 128, 16442–16443. [Google Scholar] [CrossRef]
- Bonduelle, C.; Vaca, B.M.; Coss, F.P.; Bourissou, D. Monomer versus Alcohol Activation in the 4-Dimethylaminopyridine-Catalyzed Ring-Opening Polymerization of Lactide and Lactic O-Carboxylic Anhydride. Chem. Eur. J. 2008, 14, 5304–5312. [Google Scholar] [CrossRef]
- Pounder, R.J.; Fox, D.J.; Barker, I.A.; Bennison, M.J.; Dove, A.P. Ring-opening polymerization of an O-carboxyanhydride monomer derived from l-malic acid. Polym. Chem. 2011, 2, 2204–2212. [Google Scholar] [CrossRef]
- Boullay, O.T.; Bonduelle, C.; Vaca, B.M.; Bourissou, D. Functionalized polyesters from organocatalyzed ROP of gluOCA, the O-carboxyanhydride derived from glutamic acid. Chem. Commun. 2008, 2008, 1786–1788. [Google Scholar]
- Zhang, Z.; Yin, L.; Xu, Y.; Tong, R.; Lu, Y.; Ren, J.; Cheng, J. Facile Functionalization of Polyesters through Thiol-yne Chemistry for the Design of Degradable, Cell-Penetrating and Gene Delivery Dual-Functional Agents. Biomacromolecules 2012, 13, 3456–3462. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, L.; Zhang, Y.; Zhang, Z.; Xu, Y.; Tong, R.; Cheng, J. Synthesis of Water-Soluble Poly(α-hydroxy acids) from Living Ring-Opening Polymerization of O-Benzyl-l-serine Carboxyanhydrides. ACS Macro Lett. 2012, 1, 441–444. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Shen, Z.Q.; Zhang, F.Y.; Zhang, Y.F. Ring Opening Polymerization of Caprolactone by rare earth Alkoxide-CCl4 systems. Polym. J. 1995, 27, 59–64. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Chen, X.H.; Shen, Y.Q.; Zhang, Y.F. Ring-Opening Polymerization of ε-Caprolactone by Rare Earth Coordination Catalysts. I. Characteristics, Kinetics, and Mechanism of ε-Caprolactone Polymerization with Nd (acac)3.3H2O-ALET3 System. J. Polym. Sci. Pol. Chem. 1994, 32, 597–603. [Google Scholar] [CrossRef]
- Evans, W.J.; Katsumata, H. Polymerization of c-Caprolactone by Divalent Samarium Complexes. Macromolecules 1994, 27, 2330–2332. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Shen, Z.Q.; Shen, J.L.; Zhang, Y.F. Characteristics and Mechanism of Caprolactone Polymerization with Rare Earth Halide Systems. Macromolecules 1996, 29, 3441–3446. [Google Scholar] [CrossRef]
- Yin, Q.; Tong, R.; Xu, Y.; Baek, K.; Dobrucki, L.; Fan, T.; Cheng, J. Drug-Initiated Ring-Opening Polymerization of O-Carboxyanhydrides for the Preparation of Anticancer Drug-Poly(O-carboxyanhydride) Nanoconjugates. Biomacromolecules 2013, 14, 920–929. [Google Scholar] [CrossRef]
- Yuan, M.L.; Li, X.H.; Xiong, C.D.; Deng, X.M. Polymerization of lactides and lactones 5. Ring-opening polymerization of e-caprolactone and dl-lactide by rare earth 2-methylphenyl samarium. Eur. Polym. J. 1999, 35, 2131–2138. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Xiong, C.D.; Li, X.H. Polymerization of Lactides and Lactones. II. Ring-Opening Polymerization of ε-Caprolactone and dl-Lactide by Organoacid Rare Earth Compounds. J. Appl. Polym. Sci. 1999, 71, 1941–1948. [Google Scholar] [CrossRef]
- Yuan, M.L.; Xiong, C.D.; Li, X.H.; Deng, X.M. Polymerization of Lactides and Lactones. III. Ring-Opening Polymerization of dl-Lactide by the (h3-C3H5)2Sm(m2-Cl)2(m3-Cl)2Mg(tmed)(m2-Cl)Mg(tmed) Complex. J. Appl. Polym. Sci. 1999, 73, 2857–2862. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Xiong, C.D.; Li, X.H. Polymerization of Lactides and Lactones. IV. Ring-Opening Polymerization of Caprolactone by Rare Earth Phenyl Compounds. J. Appl. Polym. Sci. 1999, 73, 1401–1408. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Li, X.H.; Xiong, C.D. Polymerization of lactides and lactones VII. Ring-opening polymerization of lactide by rare earth phenyl compounds. Eur. Polym. J. 2000, 36, 151–1156. [Google Scholar]
- Duda, A.; Penczek, S. Polymerization of E-Caprolactone Initiated by Aluminum Isopropoxide Trimer andor Tetramer. Macromolecules 1995, 28, 5981. [Google Scholar] [CrossRef]
- Tian, D.; Dubois, P.H.; Jerome, R.; Teyssie, P. Macromolecular Engineering of Polylactones and Polylactides. 18. Synthesis of Star-Branched Aliphatic Polyesters Bearing Various Functional End Groups. Macromolecules 1994, 27, 4134–4144. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the l-lactic acid O-carboxyanhydrides and poly(l-lactide acid) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, Z.; Jiang, L.; Chuan, Y.; Li, H.; Yuan, M. Ring-Opening Polymerization of l-Lactic Acid O-Carboxyanhydrides Initiated by Alkoxy Rare Earth Compounds. Molecules 2013, 18, 12768-12776. https://doi.org/10.3390/molecules181012768
He Z, Jiang L, Chuan Y, Li H, Yuan M. Ring-Opening Polymerization of l-Lactic Acid O-Carboxyanhydrides Initiated by Alkoxy Rare Earth Compounds. Molecules. 2013; 18(10):12768-12776. https://doi.org/10.3390/molecules181012768
Chicago/Turabian StyleHe, Zhengguo, Lin Jiang, Yongming Chuan, Hongli Li, and Minglong Yuan. 2013. "Ring-Opening Polymerization of l-Lactic Acid O-Carboxyanhydrides Initiated by Alkoxy Rare Earth Compounds" Molecules 18, no. 10: 12768-12776. https://doi.org/10.3390/molecules181012768





