Synthesis of Laboratory Ultrasound Contrast Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results








2.2. Discussion

3. Experimental
3.1. Internal Gas: Perfluorobutane
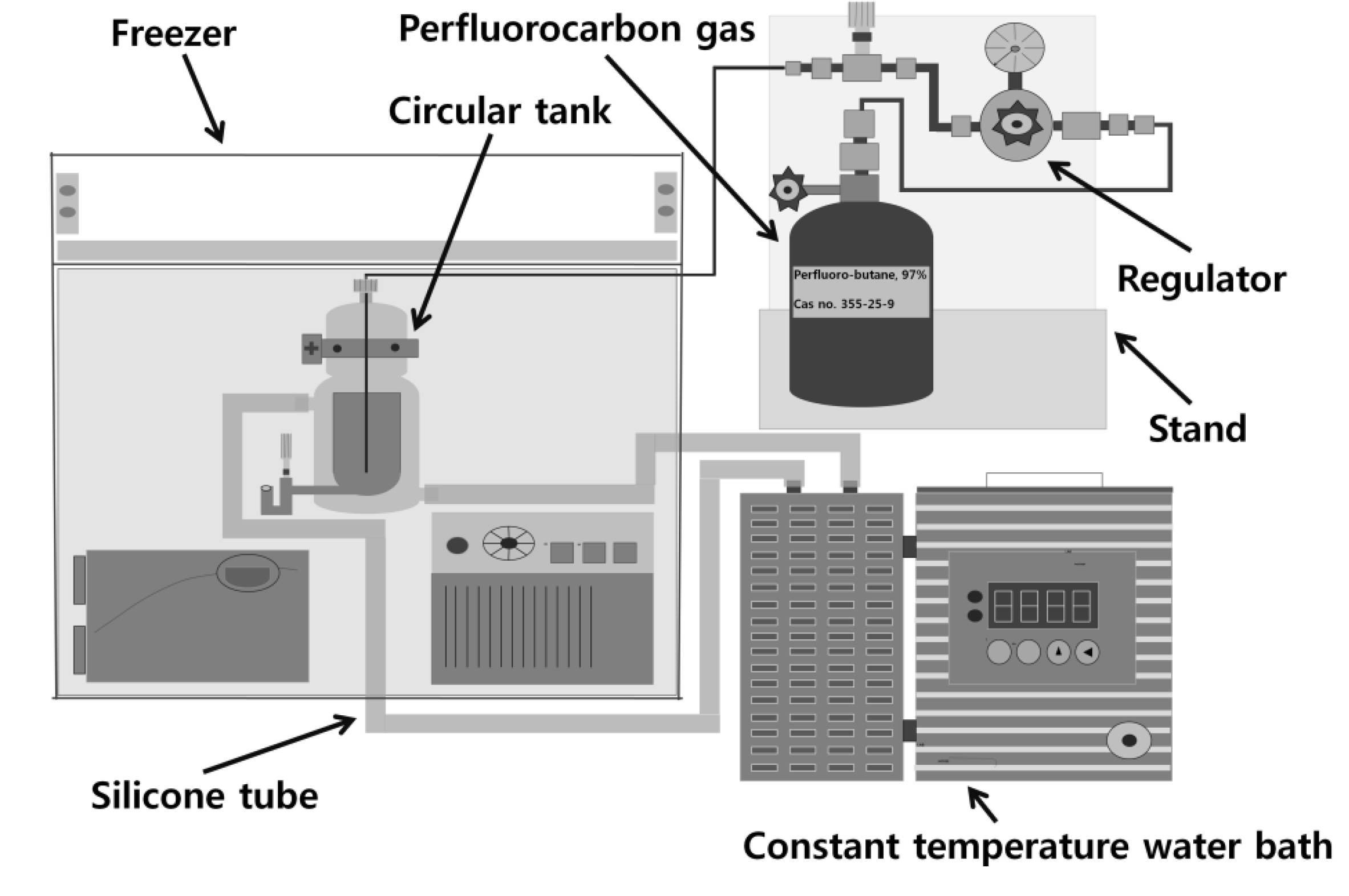
3.2. Albumin Shell Bubble Synthesis
3.3. Lipid Shell Bubble Synthesis
3.4. Analysis of UCAs


4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Correas, J.; Bridal, L.; Lesavre, A.; Mejean, A.; Claudon, M.; Helenon, O. Ultrasound contrast agent: Properties, Principles of action, tolerance and artifacts. Eur. Radiol. 2001, 11, 1316–1328. [Google Scholar] [CrossRef]
- Gramiak, R.; Shah, P.M. Echocardiography of the aortic root. Invest. Radiol. 1968, 3, 356–566. [Google Scholar] [CrossRef]
- Frinking, P.J.; Bouakz, A.; Kirhorn, J.; Ten Cate, F.J.; de Jong, N. Ultrasound contrast imaging: Current and new potential methods. Ultrasound Med. Biol. 2000, 26, 965–675. [Google Scholar] [CrossRef]
- Choi, M. Application of ultrasound in medicine: Therapeutic ultrasound and ultrasound contrast agent. The Korean society for noise and vibration engineering bimonthly. J. KSNVE 2000, 10, 743–759. [Google Scholar]
- Crum, L.A.; Fowlkes, J.B. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature 1986, 319, 52–54. [Google Scholar] [CrossRef]
- Forsberg, F.; Shi, W.T.; Goldberg, B.B. Subharmonic imaging of contrast agents. Ultrasonics 2000, 38, 93–98. [Google Scholar] [CrossRef]
- Andrew, W. Introduction to biomedical imaging. Med. Phys. 2003, 30, 107–153. [Google Scholar]
- Li, P.; Cao, L.Q.; Dou, C.Y.; Armstrong, W.F.; Miller, D.L. Impact of myocardial contrast echocardiography on vascular permeability: An in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med. Biol. 2003, 29, 1341–1349. [Google Scholar] [CrossRef]
- Li, P.; Armstrong, W.F.; Miller, D.L. Impact of myocardial contrast echocardiography on vascular permeability: Comparison of three different contrast agents. Ultrasound Med. Biol. 2004, 30, 83–91. [Google Scholar] [CrossRef]
- Burns, P. Harmonic imaging with ultrasound contrast agent. Clin. Radiol. 1996, 51, 50–55. [Google Scholar]
- Abramowicz, J.S. Ultrasound contrast media and their use in obstertrics and gynecology. Ultrasound Med. Biol. 1997, 23, 1287–1298. [Google Scholar] [CrossRef]
- Shankar, P.M.; Krishna, P.D.; Newhouse, V.L. Advantages of subharmonic over second harmonic backscatter for contrast-to-tissue echo enhancement. Ultrasound Med. Biol. 1998, 24, 395–399. [Google Scholar] [CrossRef]
- Everbach, E.C.; Makin, I.R.; Francis, C.W.; Meltzer, R.S. Effect of acoustic cavitation on platelets in the presence of an echo-contrast agent. Ultrasound Med. Biol. 1998, 24, 129–136. [Google Scholar] [CrossRef]
- Qin, S.; Caskey, C.F.; Ferrara, K.W. Ultrasound contrast microbubbles in imaging and therapy: Physical principles and engineering. Phys. Med. Biol. 2009, 54, 27–57. [Google Scholar] [CrossRef]
- Klibanov, A.L. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. 1999, 37, 139–157. [Google Scholar] [CrossRef]
- Goldberg, B.B.; Raichen, J.S.; Forsberg, F. Ultrasound Contrast Agent: Basic Principles and Clinical Application, 2nd ed.; CRC Press: London, UK, 2001. [Google Scholar]
- Mesiwala, A.H. High-intensity focused ultrasound selectively disrupts the blood brain barrier in vivo. Ultrasound Med. Biol. 2002, 28, 389–400. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubble in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef]
- Jonathan, R.; Lindner, M.D. Targeted ultrasound contrast agents: Diagnostic and therapeutic potential. IEEE Ultrason. Symp. 2001, 2, 1695–1703. [Google Scholar]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted disruption of the blood-brain barrier with focused ultrasound: Association with cavitation activity. Phys. Med. Biol. 2006, 51, 793–807. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Encapsulated ultrasound microbubbles: Therapeutic application in drug/gene delivery. J. Controlled Release 2006, 114, 89–99. [Google Scholar] [CrossRef]
- Ferrara, K.W.; Borden, M.A.; Zhang, H. Lipid-shelled vehicles: Engineering for ultrasound molecular imaging and drug delivery. Acc. Chem. Res. 2009, 42, 881–892. [Google Scholar] [CrossRef]
- Skyba, D.M.; Price, R.J.; Linka, A.Z.; Skalak, T.C.; Kaul, S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation 1998, 98, 290–293. [Google Scholar] [CrossRef]
- Jong, N.; Frinking, P.J.; Bouakaz, A.; Cate, F.J. Detection procedures of ultrasound contrast agents. Ultrasonic 2000, 38, 87–92. [Google Scholar] [CrossRef]
- Ferrara, K.W.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Cavalieri, F.; Zhou, M.; Ashokkumar, M. The design of multifunctional microbubbles for ultrasound image-guided cancer therapy. Curr. Top. Med. Chem. 2010, 10, 1198–1210. [Google Scholar] [CrossRef]
- Rapoport, N.; Gao, Z.; Kennedy, A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 2007, 99, 1095–1106. [Google Scholar] [CrossRef]
- Lindner, J.R.; Song, J.; Christiansen, J.; Klibanov, A.L.; Xu, F.; Ley, K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-Selectin. Circulation 2001, 104, 2107–2112. [Google Scholar] [CrossRef]
- Schumann, P.A,; Christiansen, J.P.; Quigley, R.M.; McCreery, T.P.; Sweizer, R.H.; Unger, E.X.; Lindner, J.R.; Matsunaga, T.O. Targeted-microbubble binding selectively to GPIIbIIIa receptors of platelet thrombi. Invest. Radiol. 2002, 37, 587–593. [Google Scholar] [CrossRef]
- Gilles, B.; Saletes, I.; Bera, J.C. Cavitation generated by amplitude modulated HIFU: Investigation on the inertial cavitation threshold. Am. Inst. Phys. 2007, 911, 171–177. [Google Scholar]
- Ay, T.; Havaux, X.; van Camp, G.; Campanelli, B.; Gisellu, G.; Pasquet, A.; Denef, J.F.; Melin, J.A.; Vanoverschelde, J.L. Destruction of contrast microbubbles by ultrasound: Effects on myocardial function, coronary perfusion pressure, and microvascular integrity. Circulation 2001, 104, 461–466. [Google Scholar] [CrossRef]
- Son, S.; Lee, S. Microfocusing using the thermal actuation of microbubbles. Microfluid. Nanofluid. 2009, 6, 77–84. [Google Scholar] [CrossRef]
- Lavon, I.; Kost, J. Ultrasound and transdermal drug delivery. Drug Discovery Today 2004, 9, 670–676. [Google Scholar] [CrossRef]
- Cavalieri, F.; Ashokkumar, M.; Grieser, F.; Caruso, F. Ultrasonic synthesis of stable, functional lysozyme microbubbles. Langmuir 2008, 24, 10078–10083. [Google Scholar] [CrossRef]
- Miller, D.L.; Gies, R.A. Gas-body-based contrast agent enhances vascular bioeffectsof 1.09MHz ultrasound on mouse intestine. Ultrasound Med. Biol. 1998, 24, 1201–1208. [Google Scholar] [CrossRef]
- Simon, R.H.; Ho, S.Y.; Lange, S.C.; Uphoff, D.F.; D'Arrigo, J.S. Application of lipid-coated microbubble ultrasonic contrast to tumor therapy. Ultrasound Med. Biol. 1993, 19, 123–125. [Google Scholar] [CrossRef]
- Klibanov, A.L. Preparation of targeted microbubbles: Ultrasound contrast agents for molecular imaging. Med. Biol. Eng. Comput. 2009, 47, 875–882. [Google Scholar] [CrossRef]
- Sirsi, S.S.; Feshitan, J.; Kwan, J.; Homma, S.; Borden, M.A. Effect of microbubble size on fundamental mode high frequency ultrasound imaging in mice. Ultrasound Med. Biol. 2010, 36, 935–948. [Google Scholar] [CrossRef]
- Quaia, E. Microbubble ultrasound contrast agents: An update. Eur. Radiol. 2007, 17, 1995–2008. [Google Scholar] [CrossRef]
- Stride, E.; Saari, N. Microbubble ultrasound contrast agents: A review. Proc. Inst. Mech. Eng. 2003, 217, 429–447. [Google Scholar] [CrossRef]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Hagisawa, K.; Tanaka, K.; Sawamura, K.; Utoguchi, N.; Nishiolka, T.; Maruyama, K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Controlled Release 2007, 117, 130–136. [Google Scholar] [CrossRef]
- Santin, M.D.; King, D.A.; Foiret, J.; Haak, A.; Obrien, W.D.; Bridal, S.L. Encapsulated contrast microbubble radial oscilation associated with postexcitation pressure peak. J. Acoust. Soc. Am. 2010, 127, 1156–1164. [Google Scholar] [CrossRef]
- Fan, C.H.; Ting, C.Y.; Lin, H.J.; Wang, C.H.; Liu, H.L.; Yen, T.C.; Yeh, C.K. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials 2013, 34, 3706–3715. [Google Scholar] [CrossRef]
- Delogu, L.G.; Vidili, G.; Venturelli, E.; Menard-Moyon, C.; Zoroddu, M.A.; Pilo, G.; Nicolussi, P.; Ligios, C.; Bedognetti, D.; Sgarrella, F.; et al. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proc. Natl. Acad. Sci. USA 2012, 109, 16612–16617. [Google Scholar] [CrossRef]
- Borden, M.A.; Qin, S.; Ferrara, K.W. Ultrasound contrast agent. Mol. Imaging 2010, 28, 425–444. [Google Scholar]
- Raisinghani, A.; DeMaria, A. Physical principles of microbubble ultrasound contrast agents. Am. J. Cardiol. 2002, 90, 3–7. [Google Scholar] [CrossRef]
- Klibanov, A.L. Microbubble contrast agents: Targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest. Radiol. 2006, 41, 354–362. [Google Scholar] [CrossRef]
- Borden, M.A.; Kruse, D.E.; Caskey, C.F.; Zhao, S.; Dayton, P.A.; Ferrara, K.W. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE 2005, 52, 1992–2002. [Google Scholar]
- Dayton, P.A.; Zhao, S.; Ferrara, K.W. Physico-chemical properties of the microbubble lipid shell ultrasound contrast agents. Ultrasonics Symp. 2004, 1, 20–23. [Google Scholar]
- Bloch, S.H.; Wan, M.; Dayton, P.A.; Ferrara, K.W. Optical observation of lipid- and polymer-shelled ultrasound microbubble contrast agents. IEEE 2004, 84, 631–633. [Google Scholar]
- Hoff, L.; Sontum, P.C.; Hovem, J.M. Oscillations of polymeric microbubbles: Effect of the encapsulating shell. J. Acoust. Soc. 2000, 107, 2272–2280. [Google Scholar] [CrossRef]
- Dagar, S.; Rubinstein, I.; Onyinste, H. Liposomes in ultrasound and gamma scintigraphic imaging. Methods Enzymol. 2000, 373, 198–214. [Google Scholar]
- Anderson, C.R.; Rychak, J.J.; Backer, M.; Backer, J.; Ley, K.; Klibanov, A.L. scVEGFmicrobubble ultrasound contrast agents: A novel probe for ultrasound molecular imaging of tumor angiogenesis. Investig. Radiol. 2010, 45, 579–585. [Google Scholar] [CrossRef]
- Goldberg, B.B.; Liu, J.B.; Forsberg, F. Ultrasound contrast agents: A review. Ultrasound Med. Biol. 1994, 20, 319–333. [Google Scholar] [CrossRef]
- Lee, Y.; Lai, C.; Li, P.; Peng, C. Ultrasound-mediated perfluorocarbonmicrobubbles bursting for gene transfection. J. Med. Biol. Eng. 2005, 25, 153–158. [Google Scholar]
- Simberg, D.; Mattrey, R. Targeting of perfluorocarbonmicrobubbles to selective populations of circulating blood cells. J. Drug Target. 2009, 17, 392–398. [Google Scholar] [CrossRef]
- Moiseyev, G.; Takahashi, Y.; Chen, Y.; Gentleman, S.; Redmond, T.M.; Crouch, R.K.; Ma, J. RPE65 Is an Iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J. Biol. Chem. 2006, 281, 2835–2840. [Google Scholar]
- Miralem, T.; Hu, Z.; Torno, M.D.; Lelli, K.M.; Maines, M.D. Small interference RNA-mediated gene silencing of human biliverdinreductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J. Biol. Chem. 2005, 280, 17084–17092. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Park, J.; Park, D.; Shin, U.; Moon, S.; Kim, C.; Kim, H.S.; Park, H.; Choi, K.; Jung, B.; Oh, J.; et al. Synthesis of Laboratory Ultrasound Contrast Agents. Molecules 2013, 18, 13078-13095. https://doi.org/10.3390/molecules181013078
Park J, Park D, Shin U, Moon S, Kim C, Kim HS, Park H, Choi K, Jung B, Oh J, et al. Synthesis of Laboratory Ultrasound Contrast Agents. Molecules. 2013; 18(10):13078-13095. https://doi.org/10.3390/molecules181013078
Chicago/Turabian StylePark, Jingam, Donghee Park, Unchul Shin, Sanghyub Moon, Chihyun Kim, Han Sung Kim, Hyunjin Park, Kiju Choi, Bongkwang Jung, Jaemin Oh, and et al. 2013. "Synthesis of Laboratory Ultrasound Contrast Agents" Molecules 18, no. 10: 13078-13095. https://doi.org/10.3390/molecules181013078




