The Novel [4,5-e][1,3]Diazepine-4,8-dione and Acyclic Carbamoyl Imino-Ureido Derivatives of Imidazole: Synthesis, Anti-Viral and Anti-Tumor Activity Evaluations
Abstract
:1. Introduction
2. Results and Discussion
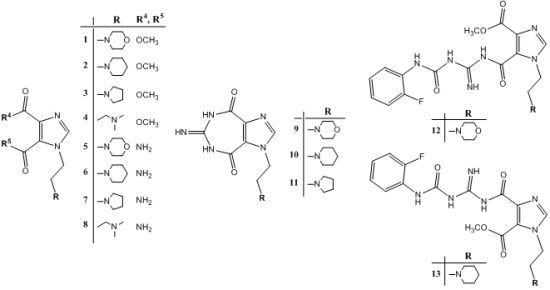
2.1. Chemistry


2.2. Structural Properties
2.3. Biological Results
2.3.2. Cytostatic Activity
| Tumor cell growth IC50a (μM) | ||||||
|---|---|---|---|---|---|---|
| Compd. | Structure | Cell lines | ||||
| MCF-7 | HepG2 | SW620 | HeLa | BJ | ||
| 1 |  | >100 | >100 | >100 | 24 | >100 |
| 2 |  | >100 | >100 | >100 | >100 | >100 |
| 3 |  | >100 | >100 | >100 | 9.5 | >100 |
| 4 |  | >100 | >100 | >100 | >100 | >100 |
| 5 |  | >100 | >100 | >100 | 32 | >100 |
| 6 |  | >100 | >100 | >100 | >100 | >100 |
| 7 |  | >100 | >100 | >100 | >100 | >100 |
| 8 |  | >100 | >100 | >100 | >100 | >100 |
| 9 |  | >100 | >100 | >100 | >100 | >100 |
| 10 |  | >100 | 41 | 31 | 31 | >100 |
| 11 |  | >100 | >100 | 20 | >100 | >100 |
| 12 |  | >100 | >100 | >100 | >100 | >100 |
3. Experimental
3.1. General Materials and Methods
3.2. Procedures for the Preparations of Compounds
3.2.1. General Procedure for Synthesis of 1–4
3.2.2. General Procedure for Synthesis of 5–8
3.2.3. General Procedure for Synthesis of 9–11
3.2.4. General Procedure for Synthesis of 12–13
3.3. Biological Methods
3.3.1. Cell Culturing
3.3.2. Proliferation Assays
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Collins, P.L.; Chancock, R.M.; Murphy, B.R. Respiratory Syncytial Virus. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 1, pp. 1443–1485. [Google Scholar]
- Anderson, L.J.; Parker, R.A.; Strikas, R.L. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J. Infect. Dis. 1990, 161, 640–646. [Google Scholar] [CrossRef]
- Meanwell, N.A.; Krystal, M. Respiratory syncytial virus: Recent progress toward the discovery of effective profylactic and therapeutic agents. Drug Discov. Today 2000, 5, 241–252. [Google Scholar] [CrossRef]
- Carter, M.C.; Alber, D.G.; Baxter, R.C.; Bithell, S.K.; Budworth, J.; Chubb, A.; Cockerill, G.S.; Dowdell, V.C.L.; Henderson, E.A.; Keegan, S.J.; et al. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. J. Med. Chem. 2006, 49, 2311–2319. [Google Scholar] [CrossRef]
- Henderson, E.A.; Alber, D.G.; Baxter, R.C.; Bithell, S.K.; Budworth, J.; Carter, M.C.; Chubb, A.; Cockerill, G.S.; Dowdell, V.C.; Fraser, I.J.; et al. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. The identification of a clinical candidate. J. Med. Chem. 2007, 50, 1685–1692. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sharma, P.K.; Kumar, N.; Dudhe, R. “Imidazoles” their chemistry and pharmacological potential. Int. J. PharmTech Res. 2011, 3, 268–282. [Google Scholar]
- Xie, M.; Ujjinamatada, R.K.; Sadowska, M.; Lapidus, R.G.; Edelman, M.J.; Hosmane, R.S. A novel, broad spectrum anti-cancer compound containing the imidazo[4,5-e][1,3]diazepine ring system. Bioorg. Med. Chem. Lett. 2010, 20, 4386–4389. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, N.; Korba, B.E.; Hosmane, R.S. Synthesis and in vitro anti-hepatitis B and C virus activities of ring-expanded ('fat') nucleobase analogues containing the imidazo[4,5-e][1,3]diazepine-4,8-dione ring system. Bioorg. Med. Chem. Lett. 2005, 15, 5397–5401. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.-M.; Sood, R.; Kalicharran, K.; Fattom, A.I.; Naso, R.B.; Barnard, D.L.; Sidwell, R.W.; Hosmane, R.S. In vitro inhibition of measles virus replication by novel ring-expanded ("fat") nucleoside analogues containing the imidazo[4,5-e][1,3]diazepine ring system. Bioorg. Med. Chem. Lett. 2002, 12, 3391–3394. [Google Scholar] [CrossRef]
- Gazivoda, T.; Raić-Malić, S.; Krištafor, V.; Makuc, D.; Plavec, J.; Bratulić, S.; Kraljević-Pavelić, S.; Pavelić, K.; Naesens, L.; Andrei, G.; et al. Synthesis, cytostatic and anti-HIV evaluations of the new unsaturated acyclic C-5 pyrimidine nucleoside analogues. Bioorg. Med. Chem. 2008, 16, 5624–5634. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–11 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wittine, K.; Poljak, K.; Kovač, M.; Makuc, D.; Plavec, J.; Balzarini, J.; Martinović, T.; Pavelić, S.K.; Pavelić, K.; Mintas, M. The Novel [4,5-e][1,3]Diazepine-4,8-dione and Acyclic Carbamoyl Imino-Ureido Derivatives of Imidazole: Synthesis, Anti-Viral and Anti-Tumor Activity Evaluations. Molecules 2013, 18, 13385-13397. https://doi.org/10.3390/molecules181113385
Wittine K, Poljak K, Kovač M, Makuc D, Plavec J, Balzarini J, Martinović T, Pavelić SK, Pavelić K, Mintas M. The Novel [4,5-e][1,3]Diazepine-4,8-dione and Acyclic Carbamoyl Imino-Ureido Derivatives of Imidazole: Synthesis, Anti-Viral and Anti-Tumor Activity Evaluations. Molecules. 2013; 18(11):13385-13397. https://doi.org/10.3390/molecules181113385
Chicago/Turabian StyleWittine, Karlo, Kristina Poljak, Matea Kovač, Damjan Makuc, Janez Plavec, Jan Balzarini, Tamara Martinović, Sandra Kraljević Pavelić, Krešimir Pavelić, and Mladen Mintas. 2013. "The Novel [4,5-e][1,3]Diazepine-4,8-dione and Acyclic Carbamoyl Imino-Ureido Derivatives of Imidazole: Synthesis, Anti-Viral and Anti-Tumor Activity Evaluations" Molecules 18, no. 11: 13385-13397. https://doi.org/10.3390/molecules181113385