Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan Films Incorporated with Carvacrol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructural Characterization


2.2. Solubility
| Films | Thickness
(mm) | Solubility
(%) | WVP
(g-mm/m2-h-kPa) | TS
(MPa) | % E | EM
(MPa) |
|---|---|---|---|---|---|---|
| CF | 0.116 ± 0.002 a | 54.57 ± 0.25 a | 1.44 ± 0.04 a | 10.169 ± 0.78 a | 8.447 ± 0.19 a | 3.958 ± 0.96 a |
| CF-CAR 0.5% | 0.111 ± 0.006 a | 31.25 ± 0.69 b | 1.09 ± 0.04 b | 7.596 ± 1.02 b | 5.474 ± 0.45 b | 2.886 ± 0.41 b |
| CF-CAR 1.0% | 0.108 ± 0.002 a | 37.63 ± 0.20 c | 1.08 ± 0.02 b | 6.384 ± 0.36 c | 4.563 ± 0.44 c | 2.638 ± 0.46 b |
| CF-CAR 1.5% | 0.112 ± 0.003 a | 39.49 ± 0.82 c | 1.13 ± 0.01 b | 7.821 ± 0.53 b | 5.820 ± 0.36 b | 3.012 ± 0.52 a |
2.3. Water Vapor Permeability (WVP)
2.4. Mechanical Properties
2.5. Optical Properties
| Films | L* | a* | b* | WI |
|---|---|---|---|---|
| CF | 59.47 ± 0.14 a | −1.30 ± 0.01 a | 14.69 ± 0.19 a | 70.44 ± 0.23 a |
| CF-CAR 0.5% | 52.59 ± 1.27 b | −0.45 ± 0.04 a | 10.09 ± 0.19 b | 68.44 ± 0.23 b |
| CF-CAR 1.0% | 54.05 ± 0.18 c | −1.42 ± 0.01 b | 12.91 ± 0.01 c | 69.52 ± 0.40 c |
| CF-CAR 1.5% | 53.07 ± 0.10 b | −1.37 ± 0.13 b | 10.02 ± 0.70 b | 69.19 ± 0.02 c |
| Films | Waveslength (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | Transparency Values | |
| CF | 0.0011 | 0.8125 | 1.41 | 14.65 | 27.80 | 31.68 | 33.50 | 34.36 | 2.49 ± 0.001 a |
| CF-CAR 0.5% | 0.0007 | 0.0019 | 5.40 | 17.30 | 30.81 | 35.46 | 38.40 | 40.40 | 2.54 ± 0.001 b |
| CF-CAR 1% | 0.0006 | 0.0018 | 1.70 | 10.23 | 24.19 | 28.08 | 29.90 | 31.00 | 2.45 ± 0.002 c |
| CF-CAR 1.5% | 0.0007 | 0.0019 | 2.90 | 13.27 | 25.48 | 28.07 | 29.31 | 29.94 | 2.44 ± 0.001 c |
2.6. Quantification of CAR Incorporated in CF

2.7. Antibacterial Activity
| Films | Inhibition Zone * (mm) | |
|---|---|---|
| S. typhimurium | E. coli O157:H7 | |
| CF | <8.0 | <8.0 |
| CF-CAR 0.5% (0.7592 g df) | <8.0 | <8.0 |
| CF-CAR 1.0% (0.8206 g df) | 9.8 ± 0.14 | <8.0 |
| CF-CAR 1.5% (0.8700 g df) | 11.7 ± 0.10 | 9.8 ± 0.16 |
2.8. Antioxidant Capacity
2.8.1. Radical Scavenging Capacity Using the DPPH Method
2.8.2. Trolox Equivalent Antioxidant Capacity (TEAC)
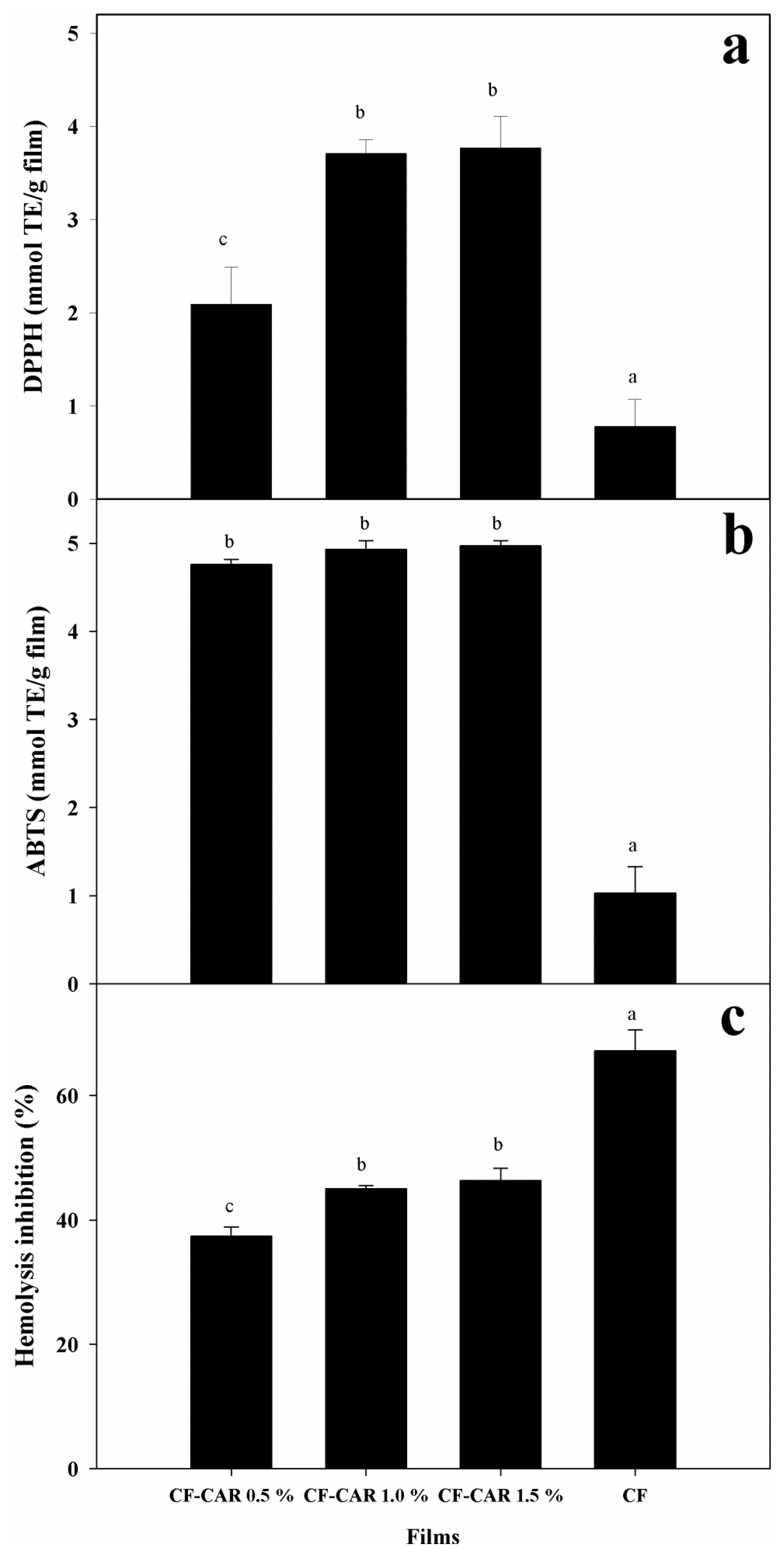
2.8.3. Evaluation of the Protective Effect on Human Erythrocytes
3. Experimental
3.1. Materials
3.2. Preparation of Chitosan
3.3. Preparation of CF with Incorporation of CAR
3.4. Thickness
3.5. Scanning Electron Microscopy
3.6. Water Solubility

3.7. Water Vapor Permeability (WVP)


3.8. Mechanical Properties
3.9. Optical Properties

3.10. Measurement of CAR Retained in Films by HPLC

3.11. Antibacterial Activity
3.12. Antioxidant Capacity
3.12.1. Radical Scavenging Capacity Using DPPH Method
3.12.2. Trolox Equivalent Antioxidant Capacity (TEAC)
3.12.3. Evaluation of Protective Effect on Human Erythrocytes

3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- No, H.; Meyers, S.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Cárdenas, G.; Anaya, P.; von Plessing, C.; Rojas, C.; Sepúlveda, J. Chitosan composite films. Biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 2397–2405. [Google Scholar] [CrossRef]
- González‐Aguilar, G.A.; Valenzuela‐Soto, E.; Lizardi‐Mendoza, J.; Goycoolea, F.; Martínez‐Téllez, M.A.; Villegas‐Ochoa, M.A.; Monroy-García, I.N.; Ayala-Zavala, J.F. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J. Sci. Food Agric. 2009, 89, 15–23. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Alvarez, I.; Niemira, B.A.; Fan, X.; Sommers, C.H. Inactivation of Salmonella enteritidis and Salmonella senftenberg in liquid whole egg using generally recognized as safe additives, Ionizing radiation, and heat. J. Food Prot. 2007, 174, 1402–1409. [Google Scholar]
- Ultee, A.; Kets, E.; Smid, E. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar]
- Del Toro-Sánchez, C.; Ayala-Zavala, J.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Namatsila, Y. Influence of chitosan and NaCl on physicochemical properties of low-acid tuna oil-in-water emulsions stabilized by non-ionic surfactant. Food Hydrocolloid. 2009, 23, 1374–1380. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/clay films properties as affected by biopolymer and clay micro/nanoparticles concentrations. Food Hydrocolloid. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Pereda, M.; Ponce, A.; Marcovich, N.; Ruseckaite, R.; Martucci, J. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocolloid. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and essential oil evaluation of a novel biodegradable film made chitosan and cinnamon with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Souza, M.P.; Teixeira, J.A.; Vicente, A.A. Physical properties of edible coatings and films made with a polysaccharide from Anacardium occidentale L. J. Food Eng. 2009, 95, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Du, W.X.; Olsen, C.; Avena-Bustillos, R.; McHugh, T.; Levin, C.; Friedman, M. Antibacterial activity against E. coli O157:H7, physical properties, and storage stability of novel carvacrol containing edible tomato films. J. Food Sci. 2008, 73, M378–M383. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of a-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocolloid. 2012, 27, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Characterization of chitosan–oleic acid composite films. Food Hydrocolloid. 2009, 23, 536–547. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the presence of glycerol and tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Jutaporn, C.T.; Suphitchaya, C.; Thawien, W. Antimicrobial activity and characteristics of edible films incorporated with Phayom wood (Shorea tolura) extract. Int. Food Res. J. 2011, 18, 39–54. [Google Scholar]
- Hoque, S.; Benjakul, S.; Propdran, T. Effects of partial hydrolysis and plasticizer content on the properties of films from cuttlefish (Sepia pharanois) skin gelatin. Food Hydrocolloid. 2011, 25, 82–90. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.; Soto-Valdez, H.; González-León, A.; Álvarez-Parrilla, E.; Martín-Belloso, O.; González-Aguilar, G. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2008, 60, 359–368. [Google Scholar] [CrossRef]
- Kurek, M.; Moundanga, S.; Favier, C.; Galić, K.; Debeaufort, F. Antimicrobial efficiency of carvacrol vapour related to mass partition coefficient when incorporated in chitosan based films aimed for active packaging. Food Control 2013, 32, 168–175. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Cerisuelo, J.P.; Gavara, R.; Hernández Muñoz, P. Preparation and characterization of chitosan/HP-β-cyclodextrins composites with high sorptions capacity for carvacrol. Carbohydr. Polym. 2013, 97, 262–268. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation; characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Liolios, C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Can-Baser, K. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Burt, S.A.; van Der Zee, R.; Koets, A.P.; de Graaff, A.M.; van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J.A. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Olasupo, N.; Fitzgerald, D.; Gasson, M.; Narbad, A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451. [Google Scholar] [CrossRef]
- Gennadios, A.; Hanna, M.; Kurth, L. Application of edible coatings on meats, poultry and seafoods: A review. LWT-Food Sci. Technol. 1997, 30, 337–350. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Youn, S.K.; Kim, Y.J.; Ahn, D.H. Antioxidative effects of chitosan in meat sausage. J. Korean Soc. Food Sci. Nutr. 2001, 30, 477–481. [Google Scholar]
- Mastelic, J.; Jerkovic, I.; Blazevic, I.; Poljak-Blazi, M.; Borovic, S.; Ivancic-Bace, I. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Eaton, P.; Nascimento, H.; Gião, M.S.; Ramos, Ó.S.; Belo, L.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X. Antioxidant activity of chitooligosaccharides upon two biological systems: Erythrocytes and bacteriophages. Carbohydr. Polym. 2010, 79, 1101–1106. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Li, Z.X.; Wang, S.; Liu, C.S.; Meng, X.H.; Liu, C.G.; Lv, Y.H.; Yu, L.J. Preparation and blood coagulation evaluation of chitosan microspheres. J. Mater. Sci. Mater. Med. 2008, 19, 1371–1377. [Google Scholar] [CrossRef]
- Hirano, S.; Zhang, M.; Nakagawa, M.; Miyata, T. Wet spun chitosan–collagen fibers, their chemical-modifications, and blood compatibility. Biomaterials 2000, 21, 997–1003. [Google Scholar] [CrossRef]
- Hongpattarake, T.; Riyaphan, O. Effect of deacetylation conditions on antimicrobial activity of chitosans prepared from carapace of black tiger shrim (Penaeus monodom). Songklanakarin J. Sci. Technol. 2008, 30, 1–9. [Google Scholar]
- López-Mata, M.A.; Ruiz-Cruz, S.; Ornelas-Paz, J.J.; Tavares-Sánchez, O.L.; Cira-Chávez, L.A.; Silva-Béltran, N.P.; Shirai, K.; Márquez-Ríos, E. Antibacterial and antioxidant properties of edible chitosan coatings incorporated with essential oils. World J. Sci. Technol. 2013, in press. [Google Scholar]
- Reed, S.J.B. Electron Microprobe Analysis and Scanning Electron Microscopy in Geology; Cambridge University Press: Cambridge, UK, 1996; pp. 173–187. [Google Scholar]
- Guillard, V.; Broyart, B.; Bonazzi, C.; Guilbert, S.; Gontard, N. Preventing moisture transfer in a composite food using edible films: Experimental and mathematical study. J. Food Sci. 2003, 68, 2267–2277. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM D882-12: Standar Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM: Philadelphia, PA, USA, 1995. [Google Scholar]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheet. 1997, 13, 287–298. [Google Scholar]
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible antimicrobial films based on chitosan matrix. J. Food Sci. 2002, 67, 1162–1169. [Google Scholar] [CrossRef]
- Ponce, A.; Fritz, R.; del Valle, C.; Roura, S. Antimicrobial activity of essential oils on the native microflora of organic. LWT-Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Moein, S.; Moein, M.R. Relationship between antioxidant properties and phenolics in Zhumeria majdae. J. Med. Plant. Res. 2010, 4, 517–521. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lu, J.; Jin, Y.; Liu, G.; Zhu, N.; Gui, M.; Yu, A.; Li, X. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 2010, 46, 205–208. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan Films Incorporated with Carvacrol. Molecules 2013, 18, 13735-13753. https://doi.org/10.3390/molecules181113735
López-Mata MA, Ruiz-Cruz S, Silva-Beltrán NP, Ornelas-Paz JDJ, Zamudio-Flores PB, Burruel-Ibarra SE. Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan Films Incorporated with Carvacrol. Molecules. 2013; 18(11):13735-13753. https://doi.org/10.3390/molecules181113735
Chicago/Turabian StyleLópez-Mata, Marco A., Saul Ruiz-Cruz, Norma P. Silva-Beltrán, José De Jesús Ornelas-Paz, Paul B. Zamudio-Flores, and Silvia E. Burruel-Ibarra. 2013. "Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan Films Incorporated with Carvacrol" Molecules 18, no. 11: 13735-13753. https://doi.org/10.3390/molecules181113735





