Glycyrrhizin Alleviates Neuroinflammation and Memory Deficit Induced by Systemic Lipopolysaccharide Treatment in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects on TNF-α and IL-1β Expressions in the Brain Tissue of LPS-Treated Mice


2.2. Effects on COX-2 and iNOS Expressions in the Brain Tissue of LPS-Treated Mice

2.3. Effect on Microglial Activation in the Hippocampal Tissue of LPS-Treated Mice

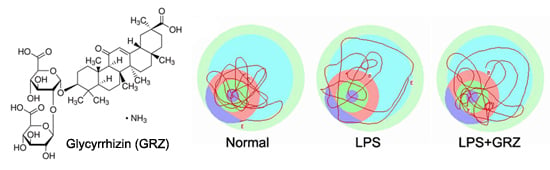
2.4. Effect on Spatial Learning of LPS-Treated Mice

2.5. Effects on Memory Deficit of LPS-Treated Mice


3. Experimental
3.1. Animals
3.2. Materials

3.3. Experimental Groups
3.4. Real-Time PCR Measurement
3.5. Western Blotting
3.6. Morris Water Maze Test
3.7. Immunohistochemistry
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sharifzadeh, M.; Shamsa, F.; Shiran, S.; Karimfar, M.H.; Miri, A.H.; Jalalizadeh, H.; Gholizadeh, S.; Salar, F.; Tabrizian, K. A time course analysis of systemic administration of aqueous licorice extract on spatial memory retention in rats. Planta Med. 2008, 74, 485–490. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, A.G. Evaluation of the immunity activity of glycyrrhizin in AR mice. Molecules 2012, 17, 716–727. [Google Scholar]
- Tabuchi, M.; Imamura, S.; Kawakami, Z.; Ikarashi, Y.; Kase, Y. The blood-brain barrier permeability of 18beta-glycyrrhetinic acid, a major metabolite of glycyrrhizin in glycyrrhiza root, a constituent of the traditional japanese medicine yokukansan. Cell. Mol. Neurobiol. 2012, 32, 1139–1146. [Google Scholar] [CrossRef]
- Wang, C.Y.; Kao, T.C.; Lo, W.H.; Yen, G.C. Glycyrrhizic acid and 18beta-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of nf-kappab through pi3k p110delta and p110gamma inhibitions. J. Agric. Food Chem. 2011, 59, 7726–7733. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, M.M.; Zhang, M.H.; Wang, R.S.; Tan, X.B.; Song, J.; Ding, S.M.; Jia, X.B.; Hu, S.Y. Protection of glycyrrhizic acid against ages-induced endothelial dysfunction through inhibiting rage/nf-kappab pathway activation in human umbilical vein endothelial cells. J. Ethnopharmacol. 2013, 148, 27–36. [Google Scholar] [CrossRef]
- Ni, Y.F.; Kuai, J.K.; Lu, Z.F.; Yang, G.D.; Fu, H.Y.; Wang, J.; Tian, F.; Yan, X.L.; Zhao, Y.C.; Wang, Y.J.; et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J. Surg. Res. 2011, 165, e29–e35. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bhattacharjee, A.; Majumder, S.; Majumdar, S.B.; Majumdar, S. Glycyrrhizic acid suppresses cox-2-mediated anti-inflammatory responses during leishmania donovani infection. J. Antimicrob. Chemoth. 2012, 67, 1905–1914. [Google Scholar] [CrossRef]
- Cherng, J.M.; Lin, H.J.; Hung, M.S.; Lin, Y.R.; Chan, M.H.; Lin, J.C. Inhibition of nuclear factor kappab is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur. J. Pharmacol. 2006, 547, 10–21. [Google Scholar] [CrossRef]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Neuroprotective Effects of glycyrrhizic acid and 18beta-glycyrrhetinic acid in pc12 cells via modulation of the pi3k/akt pathway. J. Agric. Food Chem. 2009, 57, 754–761. [Google Scholar]
- Kim, S.W.; Jin, Y.; Shin, J.H.; Kim, I.D.; Lee, H.K.; Park, S.; Han, P.L.; Lee, J.K. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting hmgb1 phosphorylation and secretion. Neurobiol. Dis. 2012, 46, 147–156. [Google Scholar] [CrossRef]
- Ohnishi, M.; Katsuki, H.; Fukutomi, C.; Takahashi, M.; Motomura, M.; Fukunaga, M.; Matsuoka, Y.; Isohama, Y.; Izumi, Y.; Kume, T.; et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology 2011, 61, 975–980. [Google Scholar] [CrossRef]
- Ni, B.; Cao, Z.; Liu, Y. Glycyrrhizin protects spinal cord and reduces inflammation in spinal cord ischemia-reperfusion injury. Int. J. Neurosci. 2013, 123, 745–751. [Google Scholar] [CrossRef]
- Gong, G.; Yuan, L.B.; Hu, L.; Wu, W.; Yin, L.; Hou, J.L.; Liu, Y.H.; Zhou, L.S. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and hmgb1. Acta Pharmacol. Sin. 2012, 33, 11–18. [Google Scholar] [CrossRef]
- Kim, J.B.; Sig Choi, J.; Yu, Y.M.; Nam, K.; Piao, C.S.; Kim, S.W.; Lee, M.H.; Han, P.L.; Park, J.S.; Lee, J.K. HMGB1, a Novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006, 26, 6413–6421. [Google Scholar] [CrossRef]
- Fang, P.; Schachner, M.; Shen, Y.Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012, 45, 499–506. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, C.; Ye, D.; Guan, D.; Ye, L.; Jin, J.; Zhao, H.; Chen, Y.; Wang, Z.; Wang, X.; et al. Diammonium glycyrrhizinate upregulates pgc-1alpha and protects against abeta1–42-induced neurotoxicity. PLoS One 2012, 7, e35823. [Google Scholar]
- Zhao, H.; Wang, S.L.; Qian, L.; Jin, J.L.; Li, H.; Xu, Y.; Zhu, X.L. Diammonium glycyrrhizinate attenuates abeta1–42-induced neuroinflammation and regulates mapk and nf-kappab pathways in vitro and in vivo. CNS Neurosci. Ther. 2013, 19, 117–124. [Google Scholar] [CrossRef]
- McGeer, E.G.; McGeer, P.L. Inflammatory processes in alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 741–749. [Google Scholar] [CrossRef]
- Shaw, K.N.; Commins, S.; O’Mara, S.M. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in bdnf expression in the rat dentate gyrus. Behav. Brain Res. 2001, 124, 47–54. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-Inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of Beta-Amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Lien, E.; Means, T.K.; Heine, H.; Yoshimura, A.; Kusumoto, S.; Fukase, K.; Fenton, M.J.; Oikawa, M.; Qureshi, N.; Monks, B.; et al. Toll-Like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 2000, 105, 497–504. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-Mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Graeber, M.B.; Streit, W.J. Microglia: Biology and pathology. Acta Neuropathol. 2010, 119, 89–105. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Hayashi, Y.; Nakanishi, H. Age-Dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience 2012, 216, 133–142. [Google Scholar] [CrossRef]
- Thomson, L.M.; Sutherland, R.J. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res. Bull. 2005, 67, 24–29. [Google Scholar] [CrossRef]
- Dantzer, R.; Bluthe, R.M.; Gheusi, G.; Cremona, S.; Laye, S.; Parnet, P.; Kelley, K.W. Molecular basis of sickness behavior. Ann. N. Y. Acad. Sci. 1998, 856, 132–138. [Google Scholar] [CrossRef]
- Hennigan, A.; Trotter, C.; Kelly, A.M. Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75ntr expression in the rat dentate gyrus. Brain Res. 2007, 1130, 158–166. [Google Scholar]
- Erickson, M.A.; Banks, W.A. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: Multiplex quantification with path analysis. Brain Behav. Immun. 2011, 25, 1637–1648. [Google Scholar] [CrossRef]
- Jeong, H.K.; Jou, I.; Joe, E.H. Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp. Mol. Med. 2010, 42, 823–832. [Google Scholar] [CrossRef]
- Park, S.M.; Choi, M.S.; Sohn, N.W.; Shin, J.W. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol. Pharm. Bull. 2012, 35, 1546–1552. [Google Scholar]
- Kudo, T.; Okamura, S.; Zhang, Y.; Masuo, T.; Mori, M. Topical application of glycyrrhizin preparation ameliorates experimentally induced colitis in rats. World J. Gastroentero. 2011, 17, 2223–2228. [Google Scholar] [CrossRef]
- Aid, S.; Bosetti, F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie 2011, 93, 46–51. [Google Scholar] [CrossRef]
- Possel, H.; Noack, H.; Putzke, J.; Wolf, G.; Sies, H. Selective upregulation of inducible nitric oxide synthase (inos) by lipopolysaccharide (lps) and cytokines in microglia: In vitro and in vivo studies. Glia 2000, 32, 51–59. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Sheng, W.; Zong, Y.; Mohammad, A.; Ajit, D.; Cui, J.; Han, D.; Hamilton, J.L.; Simonyi, A.; Sun, A.Y.; Gu, Z.; et al. Pro-Inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA(2)-IIA expression in astrocytes and microglia. J. Neuroinflamm. 2011, 8, 121. [Google Scholar] [CrossRef]
- Alvarez, A.; Cacabelos, R.; Sanpedro, C.; Garcia-Fantini, M.; Aleixandre, M. Serum TNF-Alpha levels are increased and correlate negatively with free igf-i in alzheimer disease. Neurobiol. Aging 2007, 28, 533–536. [Google Scholar] [CrossRef]
- Belarbi, K.; Jopson, T.; Tweedie, D.; Arellano, C.; Luo, W.; Greig, N.H.; Rosi, S. TNF-Alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflamm. 2011. [Google Scholar] [CrossRef]
- Wu, M.D.; Montgomery, S.L.; Rivera-Escalera, F.; Olschowka, J.A.; O’Banion, M.K. Sustained IL-1beta expression impairs adult hippocampal neurogenesis independent of il-1 signaling in nestin+ neural precursor cells. Brain Behav. Immun. 2013, 32, 9–18. [Google Scholar] [CrossRef]
- Mishra, A.; Kim, H.J.; Shin, A.H.; Thayer, S.A. Synapse loss induced by interleukin-1beta requires pre- and post-synaptic mechanisms. J. Neuroimmune Pharm. 2012, 7, 571–578. [Google Scholar] [CrossRef]
- Imamura, Y.; Wang, H.; Matsumoto, N.; Muroya, T.; Shimazaki, J.; Ogura, H.; Shimazu, T. Interleukin-1beta causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience 2011, 187, 63–69. [Google Scholar] [CrossRef]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A Dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef]
- Liu, M.C.; Liu, X.Q.; Wang, W.; Shen, X.F.; Che, H.L.; Guo, Y.Y.; Zhao, M.G.; Chen, J.Y.; Luo, W.J. Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS One 2012, 7, e43924. [Google Scholar]
- Cunningham, A.J.; Murray, C.A.; O’Neill, L.A.; Lynch, M.A.; O’Connor, J.J. Interleukin-1 beta (il-1 beta) and tumour necrosis factor (tnf) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci. Lett. 1996, 203, 17–20. [Google Scholar] [CrossRef]
- Kotilinek, L.A.; Westerman, M.A.; Wang, Q.; Panizzon, K.; Lim, G.P.; Simonyi, A.; Lesne, S.; Falinska, A.; Younkin, L.H.; Younkin, S.G.; et al. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain 2008, 131, 651–664. [Google Scholar] [CrossRef]
- Udayabanu, M.; Kumaran, D.; Nair, R.U.; Srinivas, P.; Bhagat, N.; Aneja, R.; Katyal, A. Nitric oxide associated with inos expression inhibits acetylcholinesterase activity and induces memory impairment during acute hypobaric hypoxia. Brain Res. 2008, 1230, 138–149. [Google Scholar]
- Smith, D.H.; Okiyama, K.; Thomas, M.J.; McIntosh, T.K. Effects of the excitatory amino acid receptor antagonists kynurenate and indole-2-carboxylic acid on behavioral and neurochemical outcome following experimental brain injury. J. Neurosci. 1993, 13, 5383–5392. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, J.-H.; Lee, J.-W.; Shim, B.; Lee, C.-Y.; Choi, S.; Kang, C.; Sohn, N.-W.; Shin, J.-W. Glycyrrhizin Alleviates Neuroinflammation and Memory Deficit Induced by Systemic Lipopolysaccharide Treatment in Mice. Molecules 2013, 18, 15788-15803. https://doi.org/10.3390/molecules181215788
Song J-H, Lee J-W, Shim B, Lee C-Y, Choi S, Kang C, Sohn N-W, Shin J-W. Glycyrrhizin Alleviates Neuroinflammation and Memory Deficit Induced by Systemic Lipopolysaccharide Treatment in Mice. Molecules. 2013; 18(12):15788-15803. https://doi.org/10.3390/molecules181215788
Chicago/Turabian StyleSong, Jeong-Ho, Ju-Won Lee, Beomsoo Shim, Chang-Yeol Lee, Sooyong Choi, Chulhun Kang, Nak-Won Sohn, and Jung-Won Shin. 2013. "Glycyrrhizin Alleviates Neuroinflammation and Memory Deficit Induced by Systemic Lipopolysaccharide Treatment in Mice" Molecules 18, no. 12: 15788-15803. https://doi.org/10.3390/molecules181215788