Fruit Coloration and Anthocyanin Biosynthesis after Bag Removal in Non-Red and Red Apples (Malus × domestica Borkh.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Color Change


2.2. Anthocyanin Content of Apples
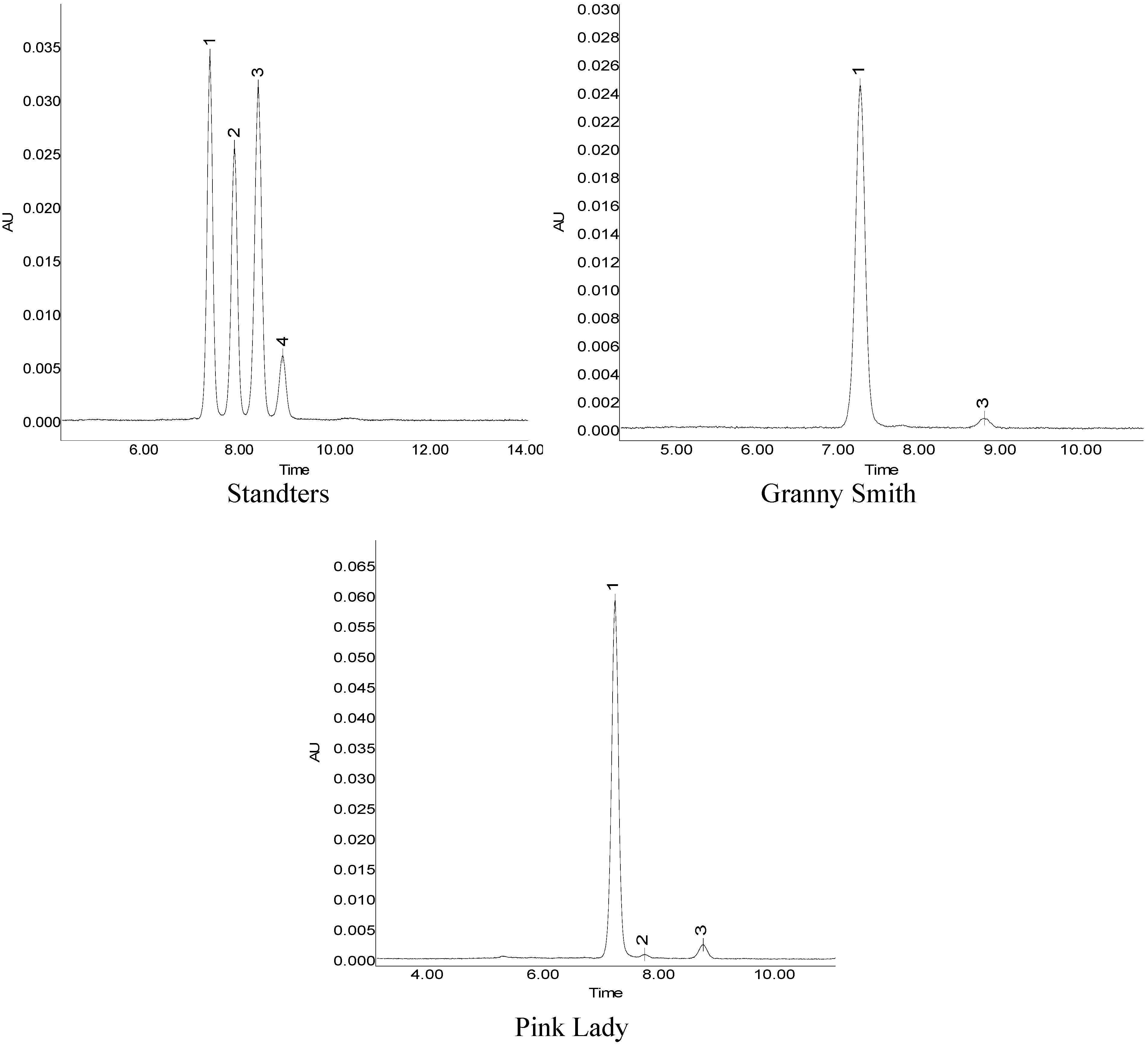
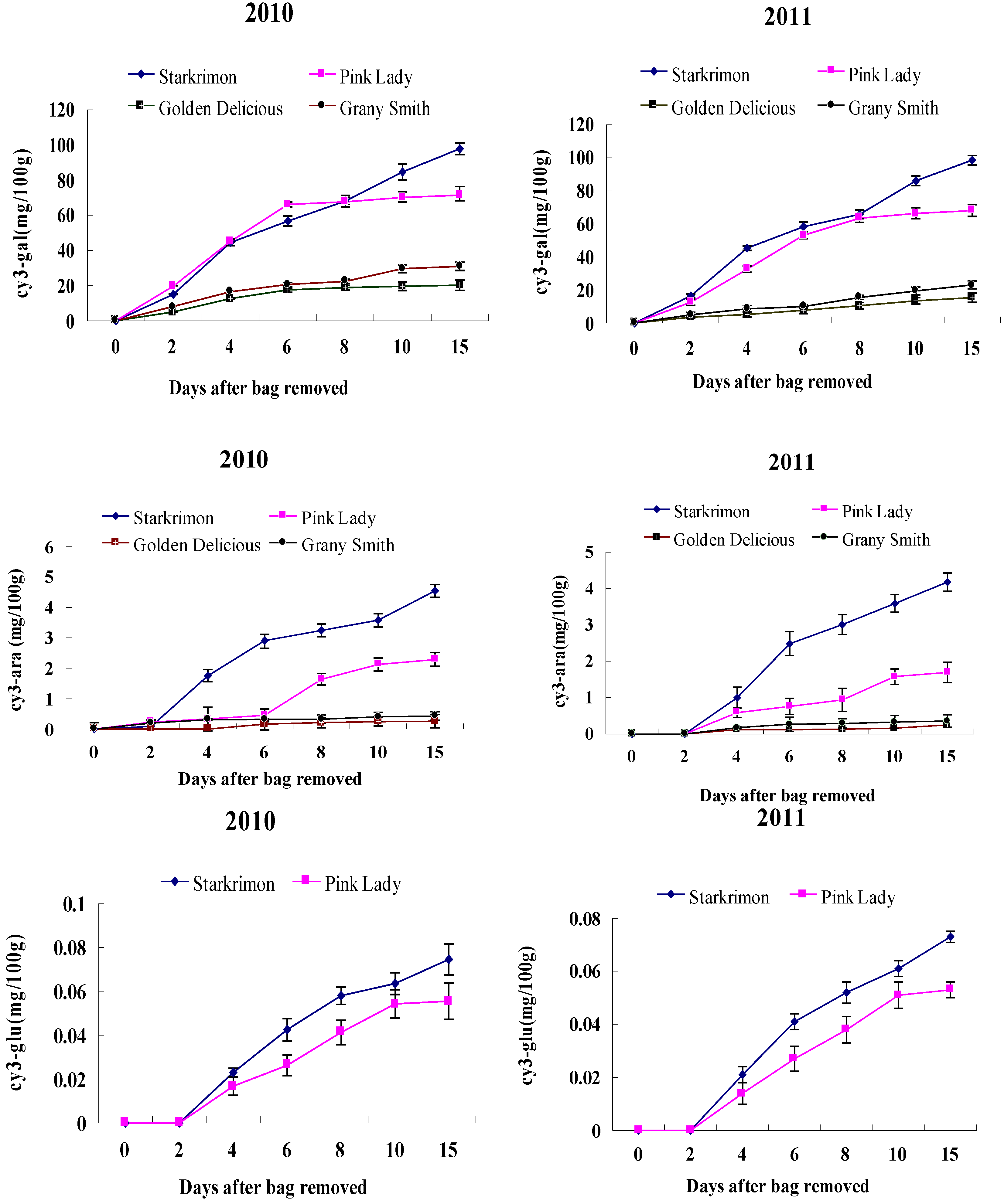
2.3. Activity of PAL, CHI, DFR, UFGT

| Cultivar | year | anthocyanin | PAL | CHI | DFR | UFGT |
|---|---|---|---|---|---|---|
| Granny Smith | 2010 | cy3-gal | 0.453 | 0.339 | 0.436 | 0.881 * |
| 2011 | 0.625 | 0.570 | 0.450 | 0.814 * | ||
| 1 | 2010 | cy3-gal | 0.529 | 0.615 | 0.271 | 0.845* |
| 2011 | 0.366 | 0.614 | 0.496 | 0.884* | ||
| Pink Lady | 2010 | cy3-gal | 0.207 | 0.793 * | 0.684 | 0.901 ** |
| 2011 | 0.365 | 0.858 * | 0.644 | 0.856 * | ||
| Starkrimon | 2010 | cy3-gal | 0.577 | 0.764 * | 0.401 | 0.865 * |
| 2011 | 0.631 | 0.827 * | 0.562 | 0.821 * |
3. Experimental
3.1. Plant Materials
3.2. Fruit Color Measurement
3.3. Determination of Enzyme Activity
3.3.1. PAL and CHI Enzyme Extraction
3.3.2. Assay of PAL Activity
3.3.3. Assay of CHI Activity
3.3.4. DFR and UFGT Enzyme Extraction
3.3.5. Assay of DFR Activity
3.3.6. Assay of UFGT Activity
3.4. Extraction, Purification, and Isolation of Anthocyanins
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the apple cultivars are available from the authors.
References
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Dougall, D.K. Regulation of skin color in apples. Crit. Rev. Plant Sci. 1992, 10, 487–502. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Ronbinson, S.P. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv Shiraz grape berries and the implications for pathway regulation. Plant Physiol. 1996, 111, 1059–1066. [Google Scholar]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red coloration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Brueggemann, J.; Weisshaar, B.; Sagasser, M. A WD40-repeat gene from Malus × domestica is a functional homologue of Arabidopsis thaliana TRANSPARENT TESTA GLABRA1. Plant Cell Rep. 2010, 29, 285–294. [Google Scholar] [CrossRef]
- Saure, M.C. External control of anthocyanin formation in apple. Sci. Hortic. 1990, 42, 181–218. [Google Scholar]
- Treutter, D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul. 2001, 34, 71–89. [Google Scholar] [CrossRef]
- Takos, A.M.; Ubi, B.E.; Robinson, S.P.; Walker, A.R. Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin. Plant Sci. 2006, 170, 487–499. [Google Scholar] [CrossRef]
- Deluc, L.; Barreu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemitry, Cell Biology, and Biotechnology. Postharvest Biol. 2001, 126, 485–493. [Google Scholar]
- Wang, H.Q.; Arakawa, O.; Motomura, Y. Influence of maturity and bagging on the relationship between anthocyanin accumulation and phenylalanine ammonia-lyase (PAL) activity in ‘Jonathan’ apples. Postharvest Biol. Technol. 2000, 19, 123–128. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J. Sci. Food Agric. 1996, 71, 313–320. [Google Scholar] [CrossRef]
- Ju, Z.G.; Yuan, Y.; Liu, C.; Wang, Y.; Tian, X. Dihydroflavonol reductase activity and anthocyanin accumulation in ‘Delicious’, ‘Golden Delicious’ and ‘Indo’ apples. Sci. Hortic. 1997, 70, 31–43. [Google Scholar]
- Ban, Y.; Kondo, S.; Ubi, B.E.; Honda, C.; Bessho, H.; Moriguchi, T. UDP-sugar biosynthetic pathway: Contribution to cyanidin 3-galactoside biosynthesis in apple skin. Planta 2009, 230, 871–881. [Google Scholar] [CrossRef]
- Iglesias, I.; Salvia, J.; Torguet, L.; Cabús, C. Orchard cooling with overtree microsprinkler irrigation to improve fruit color and quality of ‘Topred Delicious’ apples. Sci. Hortic. 2002, 93, 39–51. [Google Scholar]
- Solovchenko, A.E.; Merzlyak, M.N.; Pogosyan, S.I. Light-induced decrease of reflectance provides an insight in the photoprotective mechanisms of ripening apple fruit. Plant Sci. 2010, 178, 281–288. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analyis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995, 7, 1085–1097. [Google Scholar]
- Wang, K.L.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagnè, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit color via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Ben-Yehudah, G.; Korchinsky, R.; Redel, G.; Ovadya, R.; Oren-Shamir, M.; Cohen, Y. Color accumulation patterns and the anthocyanin biosynthetic pathway in ‘Red Delicious’ apple variants. J. Hortic. Sci. Biotech. 2005, 80, 187–192. [Google Scholar]
- Whale, S.K.; Singh, Z. Endogenous ethylene and color development in the skin of ‘Pink Lady’ apple. J. Am. Soc. Hortic. Sci. 2007, 132, 20–28. [Google Scholar]
- Rudell, D.R.; Mattheis, J.P.; Curry, E.A. Prestorage ultraviolet-white light irradiation alters apple peel metabolome. J. Agric. Food Chem. 2008, 56, 1138–1147. [Google Scholar] [CrossRef]
- Kondo, S.; Maeda, M.; Kobayashi, S.; Honda, C. Expression of anthocyanin biosynthetic genes in Malus sylvestris L. ‘Mutsu’ non-red apples. J. Hortic. Sci. Biotech. 2002, 77, 718–723. [Google Scholar]
- Ju, Z.G. Friut bagging, a useful method for studying anthocyanin synthesis and gene m expression in apple. Sci. Hortic. 1998, 77, 155–164. [Google Scholar]
- Ritenour, M.; Schrader, L.; Kammereck, R.; Donahue, R.; Edwards, G. Bag and liner color greatly affect apple temperature under full sunlight. HortScience 1997, 32, 474. [Google Scholar]
- Kim, S.-H.; Lee, J.-R.; Hong, S.-T.; Yoo, Y.-K.; An, G.; Kim, S.-R. Molecular cloning and analysis of anthocyanin biosynthesis genes preferentially expressed in apple skin. Plant Sci. 2003, 165, 403–413. [Google Scholar] [CrossRef]
- Sparvoli, F.; Martin, C.; Scienza, A.; Gavazzi, G.; Tonelli, C. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol. Biol. 1994, 24, 743–755. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar]
- Ju, Z.G.; Yuan, Y.B.; Liou, L.C.; Xin, S.H. Relationships among phenylalanine ammonia lyase activity, simple phenol concentration and anthocyanin accumulation in apple. Sci. Hortic. 1995, 61, 215–226. [Google Scholar]
- Kubo, Y. Color development of 4 apple cultivars grown in the Southeast of Japan, with special reference for bagging. J. Jpn. Soc. Hortic. Sci. 1998, 57, 191–199. [Google Scholar]
- Stafford, H.A. Flavonoid Metabolism; CRC Press: Boca Raton, FL, USA, 1990; p. 298. [Google Scholar]
- Fukuchi-Mizutani, M.; Okuhara, H.; Fukui, Y.; Nakao, M.; Katsumoto, Y.; Yonekura-Sakakibara, K.; Kusumi, T.; Hase, T.; Tanaka, Y. Biochemical and molecular characterization of a novel UDP-glucose: Anthocyanin 3'-O-Glucosyltransferase, a key enzyme for blue anthocyanin biosynthesis, from Gentian. Plant Physiol. 2003, 132, 1652–1663. [Google Scholar] [CrossRef]
- Feng, S.Q.; Chen, X.S.; Zhang, C.Y.; Liu, X.J.; Liu, Z.C.; Wang, H.B.; Wang, Y.L.; Zhou, C.H. Relationship between anthocyanin biosynthesis and related enzymes activity in Pyrus pyrifolia mantianhong and its bud sports aoguan. Sci. Agric. Sin. 2008, 7, 1318–1323. [Google Scholar]
- Han, Y.P.; Vimolmangkang, S.; Soria-Guerra, R.E.; Korban, S.S. Introduction of apple ANR genes into tobacco inhibits expression of both CHI and DFR genes in flowers, leading to loss of anthocyanin. J. Exp. Bot. 2012, 63, 2437–2447. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Che, F.; Wang, L.; Meng, R.; Zhang, X.; Zhao, Z. Fruit Coloration and Anthocyanin Biosynthesis after Bag Removal in Non-Red and Red Apples (Malus × domestica Borkh.). Molecules 2013, 18, 1549-1563. https://doi.org/10.3390/molecules18021549
Liu Y, Che F, Wang L, Meng R, Zhang X, Zhao Z. Fruit Coloration and Anthocyanin Biosynthesis after Bag Removal in Non-Red and Red Apples (Malus × domestica Borkh.). Molecules. 2013; 18(2):1549-1563. https://doi.org/10.3390/molecules18021549
Chicago/Turabian StyleLiu, Yulian, Fei Che, Lixin Wang, Rui Meng, Xiaojun Zhang, and Zhengyang Zhao. 2013. "Fruit Coloration and Anthocyanin Biosynthesis after Bag Removal in Non-Red and Red Apples (Malus × domestica Borkh.)" Molecules 18, no. 2: 1549-1563. https://doi.org/10.3390/molecules18021549





