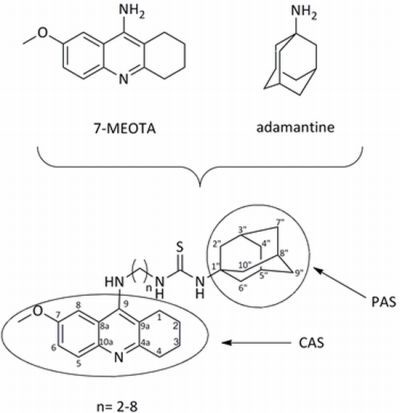
7-Methoxytacrine-Adamantylamine Heterodimers as Cholinesterase Inhibitors in Alzheimer’s Disease Treatment — Synthesis, Biological Evaluation and Molecular Modeling Studies
Abstract
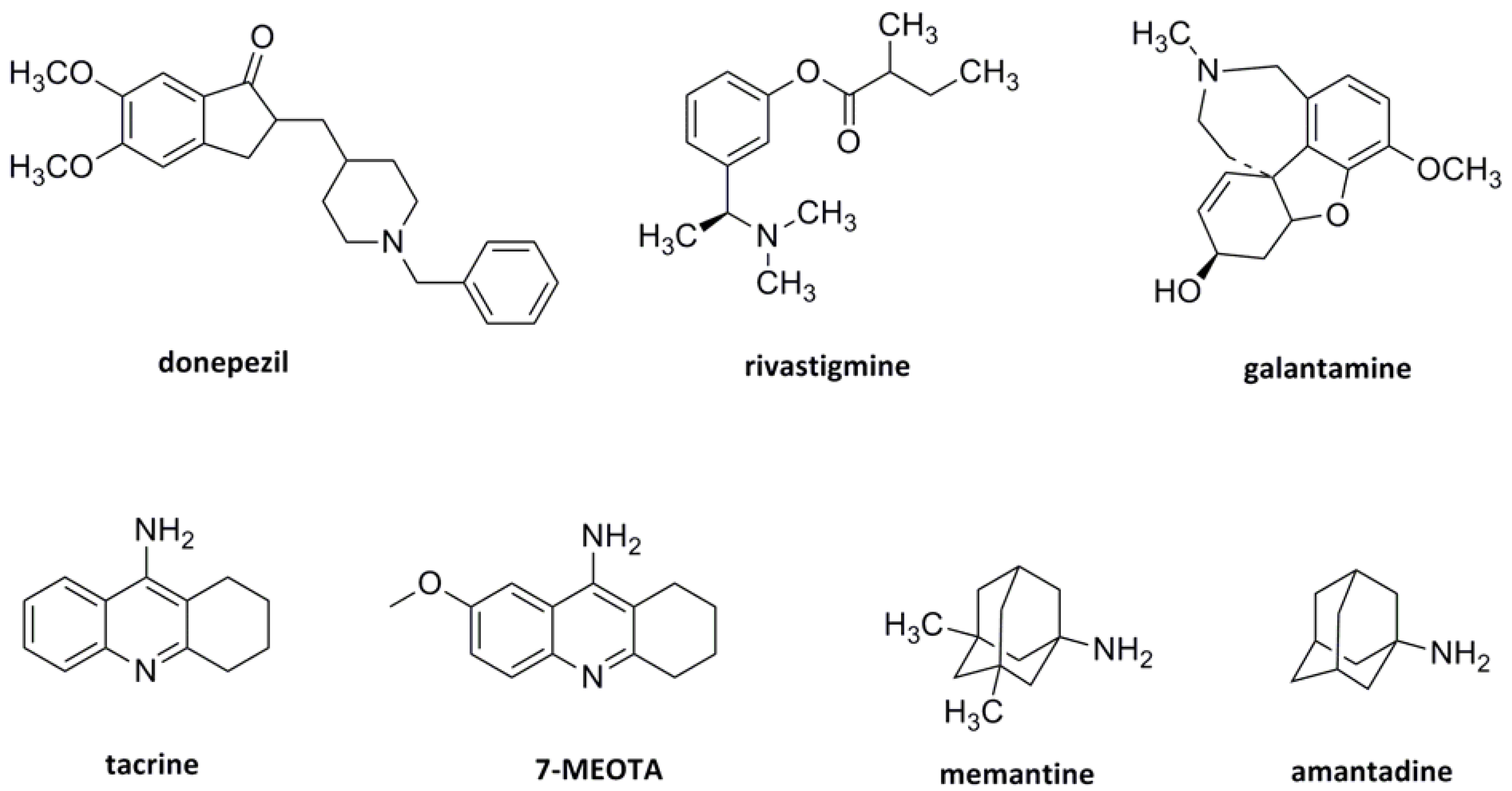
:1. Introduction
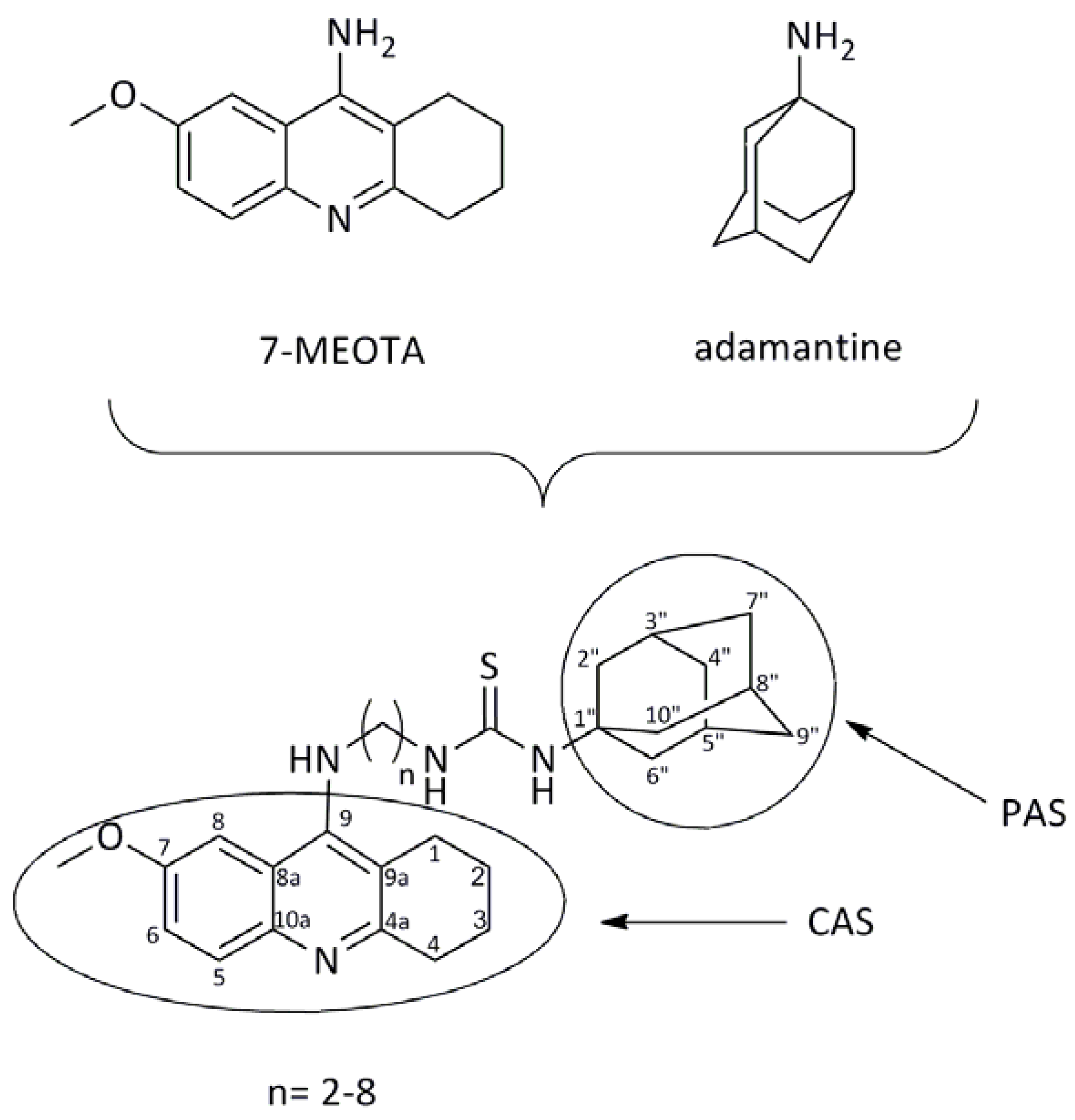
2. Results and Discussion
| Compound | IC50 (µM) ± SD b | SI c | |
|---|---|---|---|
| h AChE | hBChE | ||
| THA | 0.5 ± 0.1 | 0.023 ± 0.003 | 0.05 |
| 7-MEOTA | 10.50 ± 2.40 | 21.0 ± 3.4 | 2.0 |
| amantadine | 16.05 ± 3.13 | 102.60 ± 17.13 | 6.4 |
| 3 a | 5.32 ± 1.04 | 64.45 ± 10.76 | 12.1 |
| 4 a | 1.93 ± 0.38 | 49.77 ± 8.31 | 25.8 |
| 5 a | 1.42 ± 0.28 | 9.22 ± 1.54 | 6.5 |
| 6 a | 3.44 ± 0.67 | 29.63 ± 4.95 | 8.6 |
| 7 a | 0.21 ± 0.04 | 10.84 ± 1.81 | 51.6 |
| 8 a | 0.86 ± 0.17 | 7.26 ± 1.21 | 8.4 |
| 9 a | 0.47 ± 0.09 | 10.08 ± 1.68 | 21.4 |
| 10 | 24.96 ± 4.87 | 96.90 ± 16.18 | 3.9 |
| 11 | 5.02 ± 0.98 | 6.02 ± 1.01 | 1.2 |
| 12 | 0.53 ± 0.10 | 1.39 ± 0.23 | 2.6 |
| 13 | 2.04 ± 0.39 | 0.98 ± 0.16 | 0.5 |
| 14 | 0.47 ± 0.09 | 0.11 ± 0.02 | 0.2 |
| 15 | 2.09 ± 0.40 | 0.33 ± 0.05 | 0.2 |
| 16 | 3.47 ± 0.67 | 0.15 ± 0.02 | 0.04 |
| 17 | 1.62 ± 0.31 | 0.26 ± 0.04 | 0.2 |

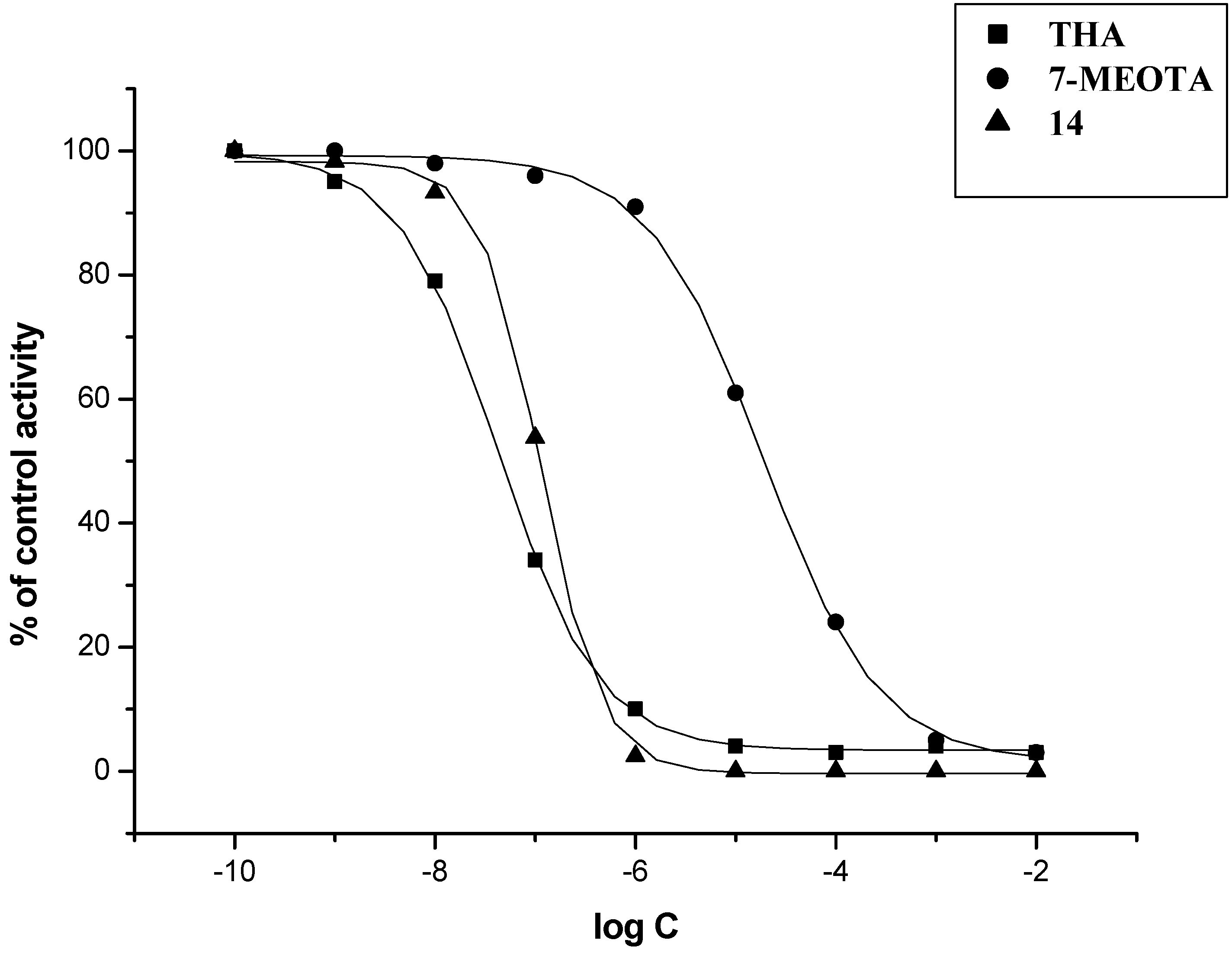

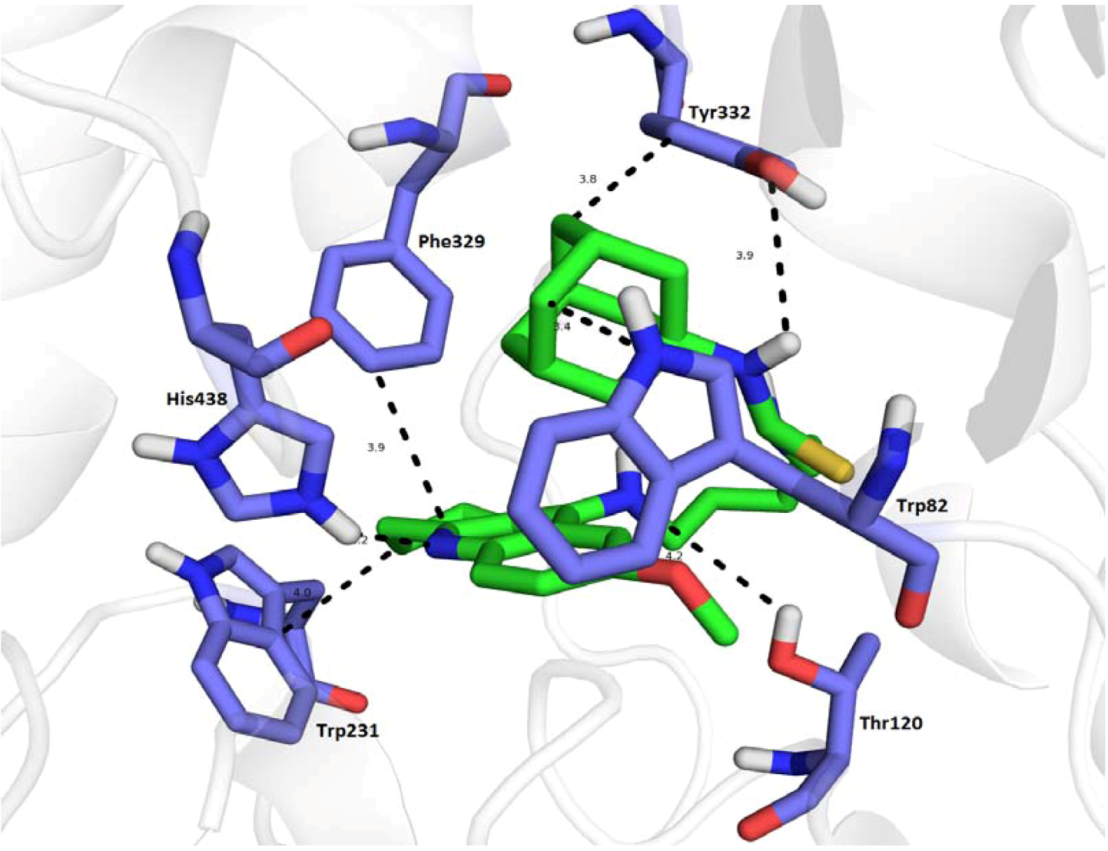
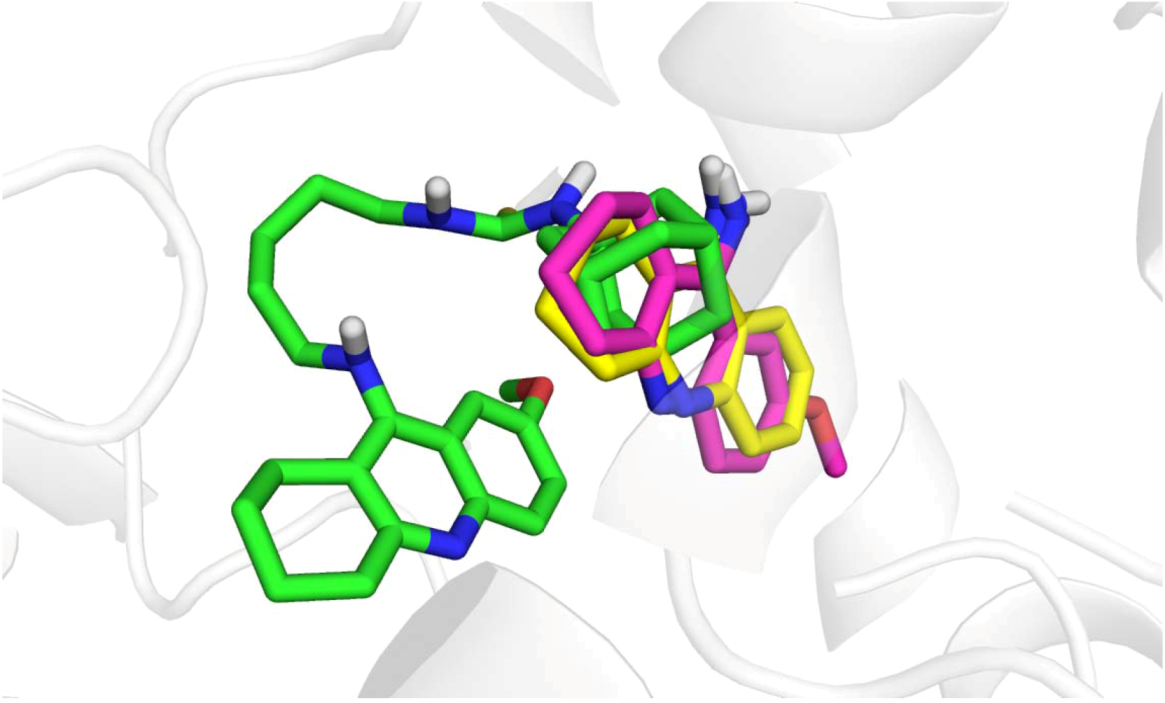
3. Experimental
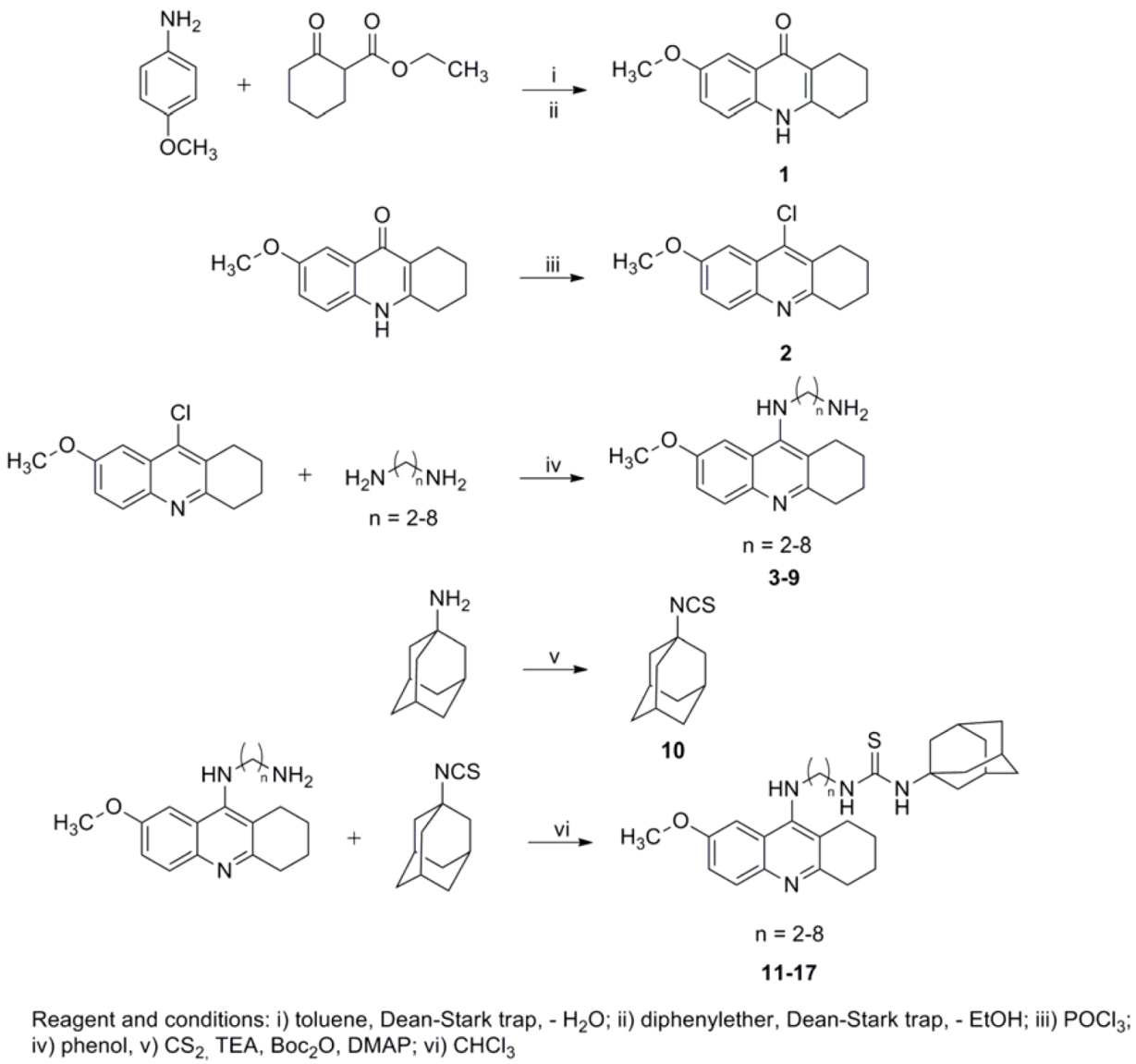
3.1. Chemistry
3.2. In Vitro Evaluation

3.3. General Procedure for Synthesis of N-(7-Methoxy-1,2,3,4-tetrahydroacridin-9-yl)alkane-1,ω-diamines (3–9)
3.4. General Procedure for Synthesis of 1-Adamantyl-3-(2-(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl-amino)alkane)thiourea 2,3-dihydroxysuccinates 11–17
3.5. Molecular Docking
4. Conclusions
Acknowledgments
References
- Lipton, S.A. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: Low-affinity, Uncompetitive antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef]
- Dominguez, E.; Chin, T.Y.; Chen, C.P.; Wu, T.Y. Management of moderate to severe Alzheimer’s disease: Focus on memantine. Taiwan J. Obstet. Gynecol. 2011, 50, 415–423. [Google Scholar] [CrossRef]
- Benzi, G.; Moretti, A. Is there a rationale for use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease? Eur. J. Pharmacol. 1998, 346, 1–13. [Google Scholar] [CrossRef]
- Walsh, D.M.; Selkoe, D.J. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 2004, 44, 181–193. [Google Scholar] [CrossRef]
- Belluti, F.; Bartolini, M.; Bottegoni, G.; Bisi, A.; Cavalli, A.; Andrisano, V.; Rampa, A. Benzophenone-based derivatives: A novel series of potent and selective dual inhibitors of acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Eur. J. Med. Chem. 2011, 46, 1682–1693. [Google Scholar] [CrossRef]
- Scarpini, E.; Scheltens, P.; Feldman, H. Treatment of Alzheimer’s disease: Current status and new perspectives. Lancet Neurol. 2003, 2, 39–47. [Google Scholar]
- Shan, W.J.; Huang, L.; Zhou, Q.; Meng, F.C.; Li, X.S. Synthesis, biological evaluation of 9-N-substituted berberine derivatives as multifunctional agents of antioxidant, inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-β aggregation. Eur. J. Med. Chem. 2011, 46, 5885–5893. [Google Scholar]
- Guo, T.; Hobbs, D.W. Development of BACE1 inhibitors for Alzheimer’s disease. 2006, 13, 1811–1829. [Google Scholar]
- Zhu, Y.; Xiao, L.; Xiong, B.; Fu, Y.; Yu, H.; Wang, W.; Wang, X.; Hu, D.; Peng, H.; Li, J.; et al. Design, Synthesis and biological evaluation of novel dual inhibitors of acetylcholinesterase and beta-secretase. Bioorg. Med. Chem. 2009, 17, 1600–1613. [Google Scholar]
- Bartus, R.T.; Dean 3rd, R.L.; Beer, B.; Lippa, A.S. Thee cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar]
- Terry, A.V., Jr.; Buccafusco, J.J. The cholinergic hypothesis age and Alzheimer’s disease- related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Whitehouse, P.J. Cholinergic therapy in dementia. Acta Neurol. Scand. Suppl. 1993, 149, 42–45. [Google Scholar]
- Kelly, C.A.; Harvey, R.J.; Cayton, H. Drug treatments for Alzheimer’s disease. BMJ 1997, 314, 693–694. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drug 2000, 60, 1095–1122. [Google Scholar] [CrossRef]
- Bielavsky, J. Analogues of 9-amino-1,2,3,4-tetrahydroacridine. Collect. Czech. Chem. Commun. 1977, 42, 2802–2808. [Google Scholar] [CrossRef]
- Yan, A.; Wang, K. Quantitative structure and bioactivity relationship study on human acetylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3336–3342. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD005593. [Google Scholar]
- McShane, R.; Areosa Sastre, A.; Minakaran, N. Memantine for dementia. Cochrane Database Syst. Rev. 2006, CD003154. [Google Scholar]
- Weiner, M.W.; Sadowsky, C.; Saxton, J.; Hofbauer, R.K.; Graham, S.M.; Yu, S.Y.; Li, S.; Hsu, H.A.; Suhy, J.; Fridman, M.; et al. Magnetic resonance imaging and neuropsychological results from a trial of memantine in Alzheimer’s disease. Alzheimers Dement. 2011, 7, 425–435. [Google Scholar]
- Munoz-Torrero, D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef]
- Chen, H.S.; Pellegrini, J.W.; Aggarwall, S.K.; Lei, S.Z.; Warach, S.; Jensen, F.E.; Lipton, S.A. Open-channel block of N-metyhl-D-aspartate (NMDA) responses by memantine: Therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992, 12, 4427–4436. [Google Scholar]
- Parson, C.G.; Danysz, W.; Quack, G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—A review of preclinical data. Neuropharmacology 1999, 38, 735–767. [Google Scholar] [CrossRef]
- Wu, H.M.; Tzeng, N.S.; Qian, L.; Wei, S.J.; Hu, X.; Chen, H.S.; Rawls, S.M.; Flood, P.; Hong, J.S.; Lu, R.B. Novel neuroprotective mechanisms of memantine: increase in neurotropic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology 2009, 34, 2344–2357. [Google Scholar]
- Kim, J.H.; Lee, H.W.; Hwang, J.; Kim, J.; Lee, M.J.; Han, H.S.; Lee, W.H.; Suk, K. Microglial-inhibiting activity of Parkinson’s disease drug amantadine. Neurobiol. Aging 2012, 33, 2145–2159. [Google Scholar] [CrossRef]
- Kornhuber, J.; Bormann, J.; Hübers, M.; Rusche, K.; Riederer, P. Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: A human postmortem brain study. Eur. J. Pharmacol. 1991, 206, 297–300. [Google Scholar] [CrossRef]
- Inzelberg, R.; Bonuccelli, U.; Schechtman, E.; Miniowich, A.; Strugatsky, R.; Ceravolo, R.; Logi, C.; Rossi, C.; Klein, C.; Rabey, J.M. Association between amantadine and the onset of dementia in Parkinson’s disease. Mor. Disord. 2006, 21, 1375–1379. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer’s disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef]
- Dejmek, L. 7-MEOTA. Drug. Future 1990, 15, 126. [Google Scholar]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–998. [Google Scholar]
- Patocka, J.; Jun, D.; Kuca, K. Possible role of hydroxylated metabolites of tacrine in drug toxicity and therapy of Alzheimer’s disease. Curr. Drug. MeTable 2008, 9, 332–335. [Google Scholar] [CrossRef]
- Korabecny, J.; Musilek, K.; Zemek, F.; Horova, A.; Holas, O.; Nepovimova, E.; Opletalova, V.; Hroudova, J.; Fisar, Z.; Jung, Y.S.; et al. Synthesis and in vitro evaluation of 7-methoxy-N-(pent-4-enyl)-1,2,3,4-tetrahydroacridin-9-amine—New tacrine derivative with cholinergic properties. Bioorg. Med. Chem. Lett. 2011, 21, 6563–6566. [Google Scholar]
- Korabecny, J.; Musilek, K.; Holas, O.; Nepovimova, E.; Jun, D.; Zemek, F.; Opletalova, V.; Patocka, J.; Dohnal, V.; Nachon, F.; et al. Synthesis and in vitro evaluation of N-(Bromobut-3-en-2-yl)-7-methoxy-1,2,3,4-tetrahydroacridin-9-amine as a cholinesterase inhibitor with regard to Alzheimer’s disease treatment. Molecules 2010, 15, 8804–8812. [Google Scholar]
- Korabecny, J.; Musilek, K.; Holas, O.; Binder, J.; Zemek, F.; Marek, J.; Pohanka, M.; Opletalova, V.; Dohnal, V.; Kuca, K. Synthesis and in vitro evaluation of N-alkyl-7-methoxytacrine hydrochlorides as potential cholinesterase inhibitors in Alzheimer disease. Bioorg. Med. Chem. Lett. 2010, 20, 6093–6095. [Google Scholar]
- Korabecny, J.; Holas, O.; Musilek, K.; Pohanka, M.; Opletalova, V.; Dohnal, V.; Kuca, K. Synthesis and in vitro evaluation of new tacrine derivatives—Bis-alkylene linked 7-MEOTA. Lett. Org. Chem. 2010, 7, 327–331. [Google Scholar] [CrossRef]
- Youdim, M.B.; Buccafusco, J.J. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol. Sci. 2005, 26, 27–35. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar]
- Kozurkova, M.; Hamulakova, S.; Gazova, Z.; Paulikova, H.; Kristian, P. Neuroactive Multifunctional Tacrine Congeners with Cholinesterase, Anti-Amyloid Aggregation and Neuroprotective Properties. Pharmaceuticals 2011, 4, 382–418. [Google Scholar] [CrossRef]
- Melchiorre, C.; Andrisano, V.; Bolognesi, M.L.; Budriesi, R.; Cavalli, A.; Cavrini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M. Acetylcholinesterase noncovalent inhibitors based on a polyamine backbone for potential use against Alzheimer’s disease. J. Med. Chem. 1998, 41, 4186–4189. [Google Scholar]
- Sterling, J.; Herzig, Y.; Goren, T.; Finkelstein, N.; Lerner, D.; Goldenberg, W.; Miskolezi, I.; Molnar, S.; Rantal, F.; Tamas, T.; et al. Novel dual inhibitors of AchE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer’s disease. J. Med. Chem. 2002, 45, 5260–5279. [Google Scholar]
- Zheng, H.; Amit, T.; Bar-Am, O.; Fridkin, M.; Youdim, M.B.; Mandel, S.A. From anti-Parkinson’s drug rasagiline to novel multitarget iron chelators with acetylcholinesterase and monoamine oxidase inhibitory and neuroprotective properties for Alzheimer’s disease. J. Alzheimers Dis. 2012, 30, 1–16. [Google Scholar]
- Weinreb, O.; Amit, T.; Bar-Am, O.; Youdim, M.B. Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain- selective monoamine oxidase inhibitory activities for Alzheimer’s disease treatment. Curr. Drug Targets 2012, 13, 483–494. [Google Scholar] [CrossRef]
- Kogen, H.; Toda, N.; Tago, K.; Marumoto, S.; Takami, K.; Ori, M.; Yamada, N.; Koyama, K.; Naruto, S.; Abe, K.; et al. Design and synthesis of dual inhibitors of acetylcholinesterase and serotonin transporter targeting potential agents for Alzheimer’s disease. Org. Lett. 2002, 4, 3359–3362. [Google Scholar]
- Toda, N.; Kaneko, T.; Kogen, H. Development of an efficient therapeutic agent for Alzheimer’s disease: Design and synthesis of dual inhibitors of acetylcholinesterase and serotonin transporter. Chem. Pharm. Bull. (Tokyo) 2011, 58, 273–287. [Google Scholar]
- Cavalli, A.; Bolognesi, M.L.; Capsoni, S.; Andrisano, V.; Bartolini, M.; Margotti, E.; Cattaneo, A.; Recanatini, M.; Melchiorre, C. A small molecule targeting the multifactorial nature of Alzheimer’s disease. Angew. Chem. Int. Ed. Engl. 2007, 46, 3689–3692. [Google Scholar]
- Hamulakova, S.; Janovec, L.; Hrabinova, M.; Kristian, P.; Kuca, K.; Banasova, M. Synthesis, Design and biological evaluation of novel highly potent tacrine congeners for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2012, 55, 23–31. [Google Scholar] [CrossRef]
- Di Santo, R.; Costi, R.; Cuzzucoli Crucitti, G.; Pescatori, L.; Rosi, F.; Scipione, L.; Celona, D.; Vertechy, M.; Ghirardi, O.; Piovesan, P.; et al. Design, Synthesis, and Structure—activity relationship of N-arylnaphtylmine derivatives as amyloid aggregation inhibitors. J. Med. Chem. 2012, 55, 8538–8548. [Google Scholar]
- Chen, Y.; Sun, J.; Fang, L.; Liu, M.; Peng, S.; Liao, H.; Lehmann, J.; Zhang, Y. Tacrine-ferulic acid-nitric oxide (NO) donor trihybrids os potent, multifunctional acetyl- and butyrylcholinesterase inhibitors. J. Med. Chem. 2012, 55, 4309–4321. [Google Scholar] [CrossRef]
- Fernandez-Bachiller, M.I.; Perez, C.; Monjas, L.; Rademann, J.; Rodriguez-Franco, M.I. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, Antioxidant, and β-amyloid-reducing. J. Med. Chem. 2012, 55, 1303–1317. [Google Scholar]
- Galdeano, C.; Viayna, E.; Sola, I.; Formosa, X.; Camps, P.; Badia, A.; Clos, M.V.; Relat, J.; Ratia, M.; Bartolini, M.; et al. Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer’s and prion disease. J. Med. Chem. 2012, 55, 661–669. [Google Scholar]
- Bolognesi, M.L.; Cavalli, A.; Valgimigli, L.; Bartolini, M.; Rosini, M.; Andrisano, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed drug design strategy: From a dual binding site acetylcholinesterase inhibitor to a trifunctional compound against Alzheimer’s disease. J. Med. Chem. 2007, 50, 6446–6449. [Google Scholar] [CrossRef]
- Stetter, H.; Wulff, C. Über Verbindungen mit Urotropin-Struktur, XXIV1) Derivative des 1-Amino-adamantans. Chem. Ber. 1962, 95, 2302–2304. [Google Scholar] [CrossRef]
- Munch, H.; Hansen, J.S.; Pittelkow, M.; Christensen, J.B.; Boas, U. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pohanka, M.; Jun, D.; Kuca, K. Improvement of acetylcholinesterase-based assay for organophosphates in way of identification by reactivators. Talanta. 2008, 77, 451–454. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.H.; Silman, I.; Sussman, J.L. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar]
- Rydberg, E.H.; Brumshtein, B.; Greenblatt, H.M.; Wong, D.M.; Shaya, D.; Williams, L.D.; Carlier, P.R.; Pang, Y.P.; Silman, I.; Sussman, J.L. Complexes of alkylene-liked tacrine dimers with Torpedo california acetylcholinesterase: Binding of Bis(5)-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem. 2006, 49, 5491–5500. [Google Scholar]
- Camps, P.; Formosa, X.; Galdeano, C.; Munoz-Torrero, D.; Ramirez, L.; Gomez, E.; Isambert, N.; Lavilla, R.; Badia, A.; Clos, M.V.; et al. Pyrano[3,2-c]quinoline-6-chlorotacrine hybrids as a novel family of acetylcholinesterase- and beta-amyloid-directed anti-Alzheimer compounds. J. Med. Chem. 2009, 52, 5365–5379. [Google Scholar]
- Fernandez-Bachiller, M.I.; Perez, C.; Campillo, N.E.; Paez, J.A.; Gonzales-Munoz, G.C.; Usan, P.; Garcia-Palomero, E.; Lopez, M.G.; Villarroya, M.; Garcia, A.G.; et al. Tacrine-melatonin hybrids as multifunctional agents for Alzheimer’s disease, with cholinergic, antioxidant, and neuroprotective properties. ChemMedChem 2009, 4, 828–841. [Google Scholar] [CrossRef]
- Haviv, H.; Wong, D.M.; Greenblatt, H.M.; Carlier, P.R.; Pang, Y.P.; Silman, I.; Sussman, J.L. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J. Am. Chem. Soc. 2005, 127, 11029–11036. [Google Scholar]
- Nicolet, Y.; Lockbridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar]
- Kryger, G.; Harel, M.; Giles, K.; Toker, L.; Velan, B.; Lazar, A.; Kronman, C.; Barak, D.; Ariel, N.; Shafferman, A.; et al. Structures of recombinant native and E202Q mutant human acetylcholinesterase complexed with the snake-venom toxin fasciculin-II. Acta Crystallogr. D Biol. Crystalogr. 2000, 56, 1385–1394. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System. Available online: http://www.pymol.org (accessed on 1 January 2002).
- Sample Availability: Samples of all prepared compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Spilovska, K.; Korabecny, J.; Kral, J.; Horova, A.; Musilek, K.; Soukup, O.; Drtinova, L.; Gazova, Z.; Siposova, K.; Kuca, K. 7-Methoxytacrine-Adamantylamine Heterodimers as Cholinesterase Inhibitors in Alzheimer’s Disease Treatment — Synthesis, Biological Evaluation and Molecular Modeling Studies. Molecules 2013, 18, 2397-2418. https://doi.org/10.3390/molecules18022397
Spilovska K, Korabecny J, Kral J, Horova A, Musilek K, Soukup O, Drtinova L, Gazova Z, Siposova K, Kuca K. 7-Methoxytacrine-Adamantylamine Heterodimers as Cholinesterase Inhibitors in Alzheimer’s Disease Treatment — Synthesis, Biological Evaluation and Molecular Modeling Studies. Molecules. 2013; 18(2):2397-2418. https://doi.org/10.3390/molecules18022397
Chicago/Turabian StyleSpilovska, Katarina, Jan Korabecny, Jan Kral, Anna Horova, Kamil Musilek, Ondrej Soukup, Lucie Drtinova, Zuzana Gazova, Katarina Siposova, and Kamil Kuca. 2013. "7-Methoxytacrine-Adamantylamine Heterodimers as Cholinesterase Inhibitors in Alzheimer’s Disease Treatment — Synthesis, Biological Evaluation and Molecular Modeling Studies" Molecules 18, no. 2: 2397-2418. https://doi.org/10.3390/molecules18022397