Simultaneous Determination of Flavonoids, Isochlorogenic Acids and Triterpenoids in Ilex hainanensis Using High Performance Liquid Chromatography Coupled with Diode Array and Evaporative Light Scattering Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Extraction and Chromatographic Conditions
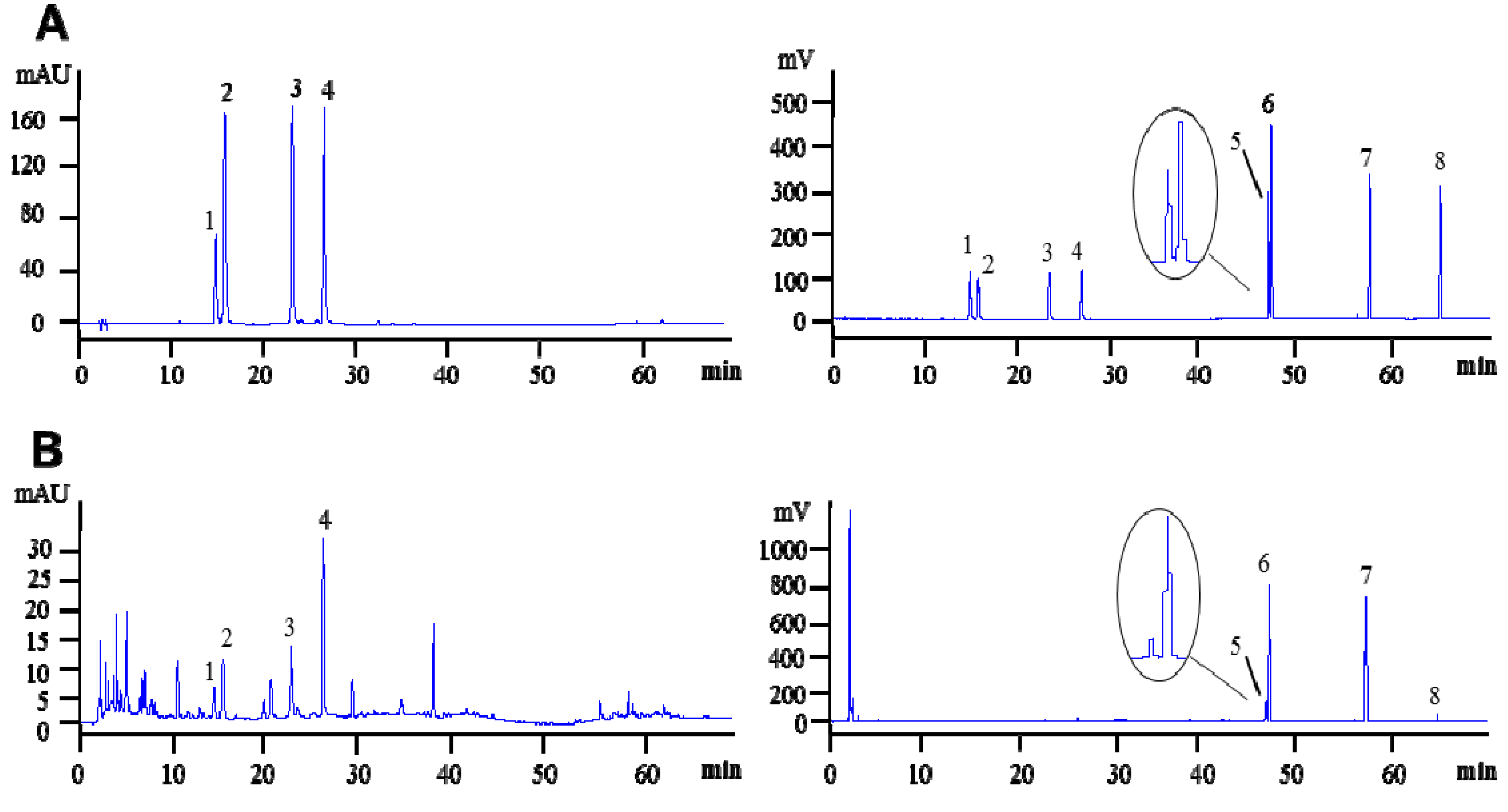
2.2. Validation of HPLC Method
| Analytes | Regressive equation | R2 | Linear range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| 1 | y = 4.839x − 1.603 | 0.9994 | 3.36–215.00 | 0.42 | 1.68 |
| 2 | y =14.732x − 7.012 | 0.9993 | 0.83–212.00 | 0.21 | 0.83 |
| 3 | y = 12.155x − 15.712 | 0.9994 | 1.69–216.00 | 0.42 | 0.84 |
| 4 | y = 11.587x − 27.040 | 0.9993 | 3.13–200.00 | 0.39 | 0.78 |
| 5 | y= 1.603x − 0.447 | 0.9993 | 13.75–330.00 | 6.88 | 13.75 |
| 6 | y = 1.627x − 0.281 | 0.9991 | 12.56–400.00 | 6.28 | 12.56 |
| 7 | y = 1.621x − 0.369 | 0.9991 | 13.13–420.00 | 6.56 | 13.13 |
| 8 | y = 1.762x − 0.782 | 0.9992 | 13.06–313.50 | 6.53 | 13.06 |
| Analytes | Precision (RSD, %, n = 6) | Repeatability (RSD, %, n = 3) | Recovery (%, n = 3) | ||||
|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | LL | ML | HL | Mean | RSD | |
| 1 | 0.6 | 1.3 | 2.0 | 0.7 | 2.6 | 98.7 | 2.8 |
| 2 | 0.8 | 1.2 | 2.4 | 3.4 | 1.9 | 97.6 | 1.3 |
| 3 | 0.8 | 1.6 | 2.4 | 1.9 | 1.7 | 96.2 | 2.7 |
| 4 | 1.4 | 1.8 | 2.3 | 3.1 | 1.6 | 100.8 | 0.7 |
| 5 | 0.8 | 1.0 | 1.1 | 1.2 | 2.4 | 94.6 | 3.5 |
| 6 | 0.7 | 0.5 | 1.8 | 1.4 | 2.1 | 103.3 | 0.4 |
| 7 | 0.9 | 1.1 | 2.1 | 2.2 | 2.2 | 98.8 | 4.1 |
| 8 | 1.5 | 1.6 | 2.4 | 2.6 | 3.2 | 98.0 | 2.8 |
2.3. Quantitative Analysis of Investigated Compounds in Ilex Hainanensis
| Samples | Compounds (n = 3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Tritepenoids (Sum of 5–8) | |
| IH1 | 2.30 | 1.34 | 9.95 | 2.45 | 9.28 | 30.69 | 7.47 | 5.64 | 53.08 |
| IH2 | 1.14 | 0.26 | 3.41 | 1.22 | 8.75 | 22.05 | 23.32 | 5.08 | 59.20 |
| IH3 | 1.13 | 0.51 | 3.18 | 1.46 | 7.72 | 18.23 | 16.07 | 3.95 | 45.97 |
| IH4 | 2.52 | 2.12 | 2.56 | 5.91 | 17.62 | 41.17 | 50.57 | 10.99 | 120.35 |
3. Experimental
3.1. Chemical Materials and Reagents

3.2. Sample Preparation
3.3. HPLC-DAD-ELSD Analysis
3.4. Calibration Curves
3.5. Limits of Detection and Quantification
3.6. Precision, Repeatability and Accuracy
4. Conclusions
Acknowledgments
References
- Pharmacopoeia Commission of PRC, Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; Volume I, p. 469.
- Yang, J. Clinical observation of left ventricular hypertrophy reversed by Shan-lü-cha tablet of different doses. Gunagxi Med. J. 2006, 28, 1375–1376. [Google Scholar]
- Hui, Y.C.; Shu, Z.Q. Clinical comparison between Shan-lü-cha tablets with Captopril on reversal of left ventricular hypertrophy. Chin. J. Integr. Tradit. West. Med. 2002, 22, 206. [Google Scholar]
- Li, P.; Xie, J.X.; Chen, Y.; Chen, J. The impact of different processed products of Shan-lü-cha on the experimental hyperlipidemia quail lipids. J. Chin. Med. Mater. 2008, 31, 1627–1630. [Google Scholar]
- Zhao, B. The application of Zhongzu Shan-lü-cha tablet in hypertensive benign nephrosclerosis. Chin. J. Mod. Drug. Appl. 2008, 2, 26–27. [Google Scholar]
- Liu, Y.; Wei, H.Y.; Long, J.C.; Jiang, Z.O. Pharmacodynamics of Shan-lü-cha antihypertensive tablets for the treatment of hypertension with vertigo and headache. Chin. J. Exp. Tradit. Med. Form 2010, 16, 86–88. [Google Scholar]
- Li, S.; Mo, W.M.; Zhang, Z.J.; Liu, L.M.; Lu, X.; Liao, X.Y. Determination the content of rutin in Shan-lü-cha by HPLC. China Pharm. 2006, 9, 807–809. [Google Scholar]
- Li, P.; Peng, B.C.; Li, S.Q.; Zhen, D.D.; C, J.; Han, B.Z. Anti-hyperglycemic effect of Ilex hainanensis extract on glycuresis in rats induced by mesoxyalyurea. Chin. J. Exp. Tradit. Med. Form. 2010, 16, 137–138. [Google Scholar]
- Chen, Y.; Xie, Z.; Liu, J.; Xin, H. Review of the researches on Ilex hainanensis. J. Guangxi. Tradit. Chin. Med. Univ. 2004, 7, 4–6. [Google Scholar]
- Wei, J.; Sun, Y.J. Effect of Hainan holly leaf total flavonoids on serum lipids based on hyperlipidemia mice. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2009, 30, 64–70. [Google Scholar]
- Sheng, S.D.; Yu, Y.J.; Wang, D.Y. Inhibitive effect of Hainan holly leaf extract on apoptosis in rats with benign prostatic hyperplasia (BPH). J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2010, 31, 53–56. [Google Scholar]
- Sheng, S.D.; Yu, Y.J.; Wang, D.Y.; Qian, X.M.; Xiao, W.S. Inhibition of Hainan holly leaf extract on rats with experimental benign prostatic hyperplasia. J. China Pharm. Univ. 2011, 42, 551–554. [Google Scholar]
- Chen, X.Q.; Zan, K.; Yang, J.; Liu, X.X.; Mao, Q.; Zhang, L.; Lai, M.X.; Wang, Q. Quantitative analysis of triterpenoids in different parts of Ilex hainanensis, Ilex stewardii and Ilex pubescens using HPLC-ELSD and HPLC-MSn and antibacterial activity. Food Chem. 2011, 126, 1454–1459. [Google Scholar]
- Shang, X.F.; Pan, H.; Li, M.X.; Miao, X.L.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar]
- Chen, X.L. Review of the researches on Lonicera japonica. J. Chongqing Coll. Ed. 2007, 20, 15–21. [Google Scholar]
- Liu, H.; Kang, J.D.; Li, J.X.; Lu, P.W. Content determination of rutin in shanglucha by HPLC. J. Henan Univ. (Med. Sci.) 2011, 30, 241–244. [Google Scholar]
- Zhao, D.F. Determination of rutin in Shanlucha Capsule by HPLC. Chin. Tradit. Pat. Med. 2002, 24, 724–725. [Google Scholar]
- Wei, X.L.; Chen, X.Q.; Yang, J.; Wang, Q. Determination of the content of ilexgenin A in Shanlücha crude drug and its preparations. Northwest Pharm. J. 2010, 25, 175–176. [Google Scholar]
- Chen, Y.; Li, Y.H.; Xie, Z.; Liu, J.; Xin, H.; Liu, B.C.; Yu, Y.C. Chlorogenic acid content comparison of different processed products of Ilex hainanensis Merr. J. Chin. Med. Mater. 2005, 28, 107–109. [Google Scholar]
- Qi, L.W.; Yu, Q.T.; Li, P.; Li, S.L.; Wang, Y.X.; Sheng, L.H.; Yi, L. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J. Chromatogr. A. 1134, 162–169.
- Chen, X.Q.; Yang, J.; Liu, X.X.; Lai, M.X.; Wang, Q. Complete assignments of 1H and 13C-NMR spectral data for three new triterpenoid saponins from Ilex hainanensis Merr. Magn. Reson. Chem. 2009, 47, 169–173. [Google Scholar]
- Chen, X.Q.; Zan, K.; Liu, H.; Yang, J.; Lai, M.X.; Wang, Q. Triterpenes and flavonoids from Ilex hainanensis Merr. (Aquifoliaceae). Biochem. Syst. Ecol. 2009, 37, 678–682. [Google Scholar]
- Ren, D.M.; Yuan, J.R. Studies on the Chemical Constituents of Dracocephalum rupestre Hance. Chin. Tradit. Herb Drugs 1997, 28, 74–76. [Google Scholar]
- Wen, D.X.; Zhen, X.Z.; Kenichiro, I. Studies on the Chemical Constituents of Ilex hainanensis Merr. Chin. J. Chin. Mater. Med. 1999, 24, 223–255. [Google Scholar]
- Sample Availability: Samples of the compounds and materials are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, B.; Qiao, C.-F.; Zhao, J.; Huang, W.-H.; Hu, D.-J.; Liu, H.-G.; Li, S.-P. Simultaneous Determination of Flavonoids, Isochlorogenic Acids and Triterpenoids in Ilex hainanensis Using High Performance Liquid Chromatography Coupled with Diode Array and Evaporative Light Scattering Detection. Molecules 2013, 18, 2934-2941. https://doi.org/10.3390/molecules18032934
Peng B, Qiao C-F, Zhao J, Huang W-H, Hu D-J, Liu H-G, Li S-P. Simultaneous Determination of Flavonoids, Isochlorogenic Acids and Triterpenoids in Ilex hainanensis Using High Performance Liquid Chromatography Coupled with Diode Array and Evaporative Light Scattering Detection. Molecules. 2013; 18(3):2934-2941. https://doi.org/10.3390/molecules18032934
Chicago/Turabian StylePeng, Bo, Chun-Feng Qiao, Jing Zhao, Wei-Hua Huang, De-Jun Hu, Hua-Gang Liu, and Shao-Ping Li. 2013. "Simultaneous Determination of Flavonoids, Isochlorogenic Acids and Triterpenoids in Ilex hainanensis Using High Performance Liquid Chromatography Coupled with Diode Array and Evaporative Light Scattering Detection" Molecules 18, no. 3: 2934-2941. https://doi.org/10.3390/molecules18032934





