Chemical Constituents from the Leaves of Annona reticulata and Their Inhibitory Effects on NO Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Compounds
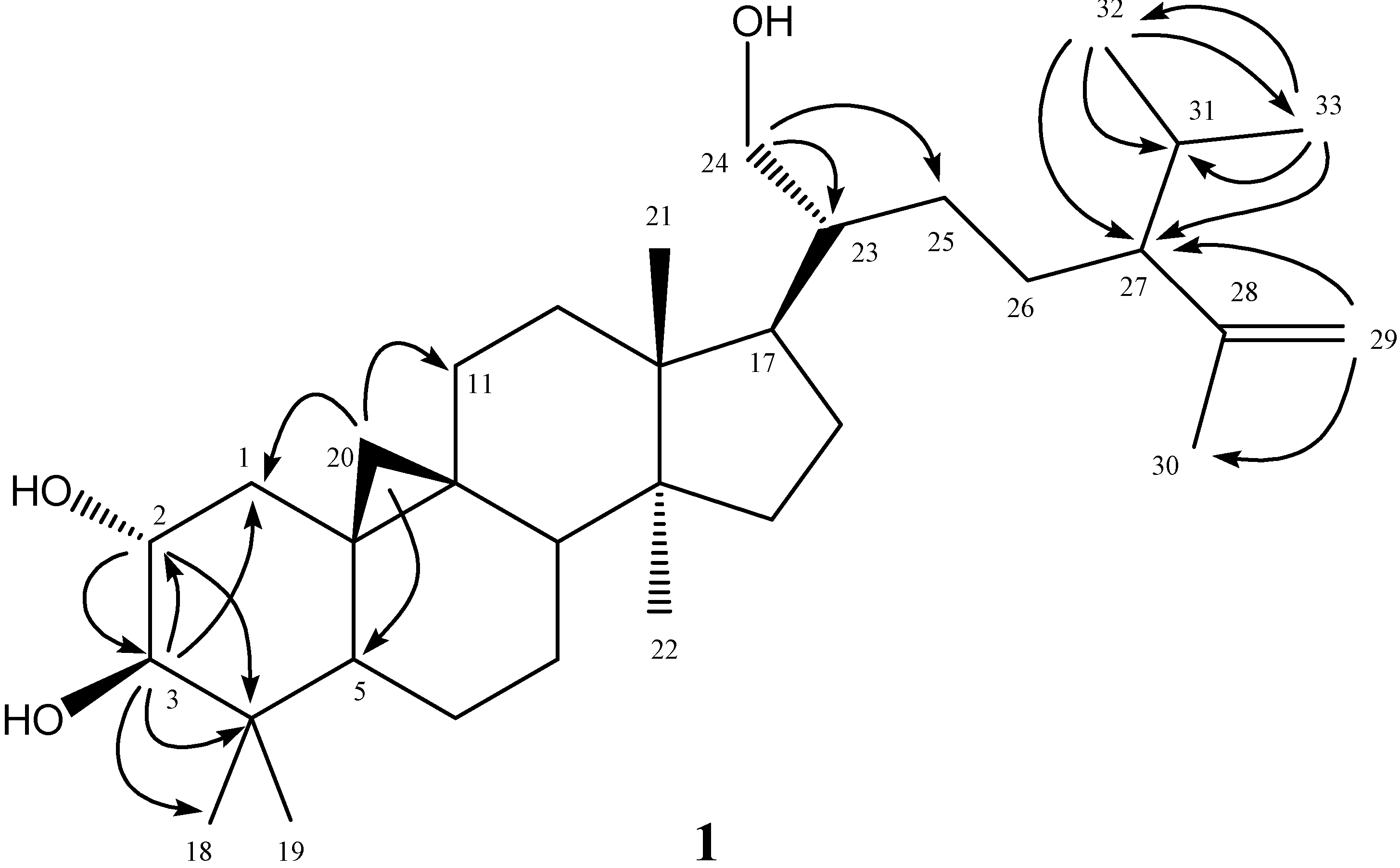
2.2. Structural Elucidation of New Compound 1
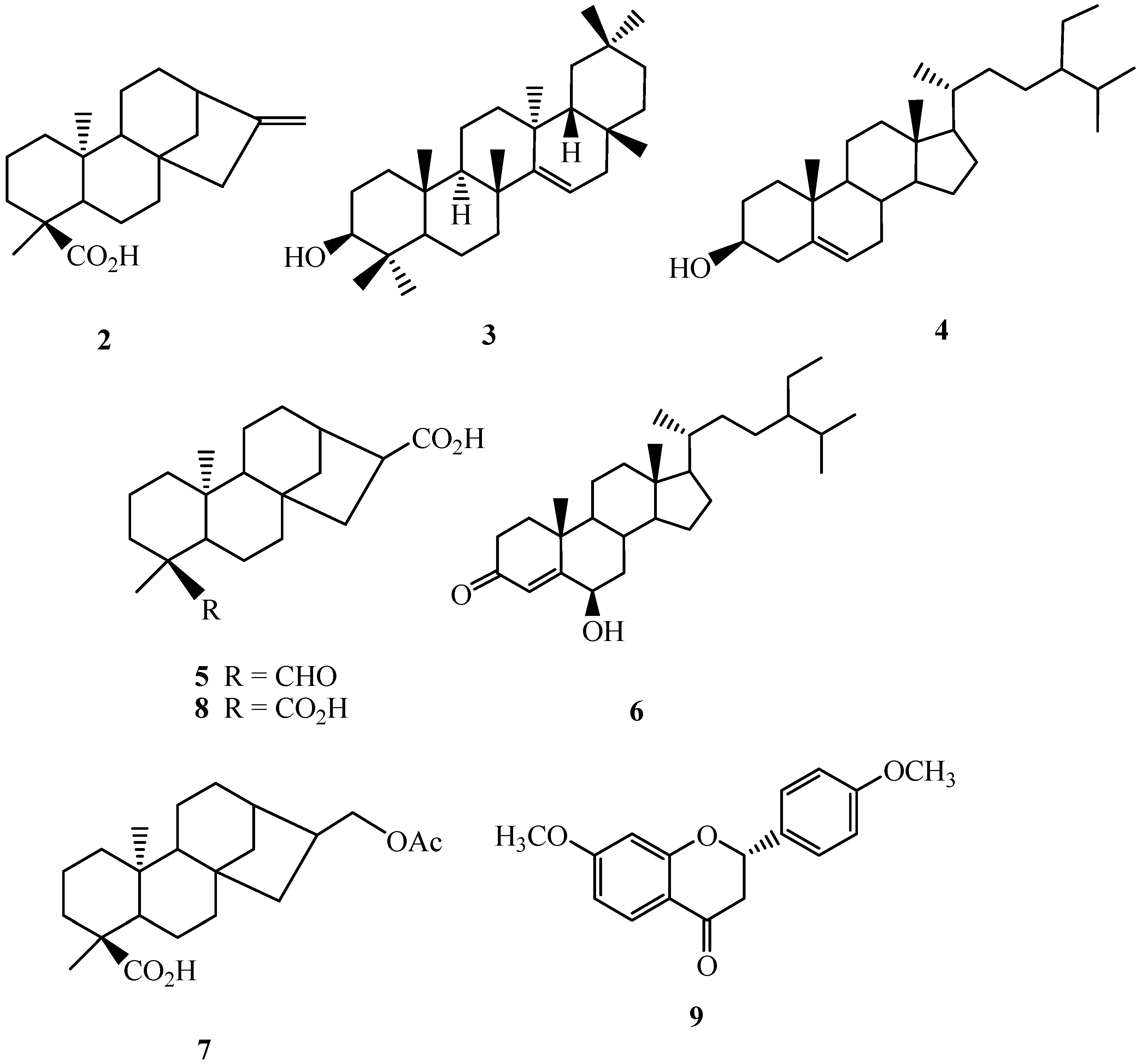
2.3. The Inhibitory Effects of Isolated Compounds on NO Production
| Dose (μM) | Cell viability(% of control) | NO level | NO inhibition(% of control) | IC50(μM) | |
|---|---|---|---|---|---|
| Control | (−) | 100.0 ± 4.9 | −0.5 ± 0.1 | (−) | (−) |
| LPS | (+) | 98.7 ± 8.0 | 45.4 ± 2.7 ### | (−) | (−) |
| 1 | 12.5 25 50 100 | 88.0 ± 1.5 81.2 ± 1.8 73.0 ± 3.6 * 66.9 ± 5.5 ** | 36.6 ± 1.9 33.5 ± 0.9 (−) (−) | 19.3 ± 4.2 26.2 ± 2.0 (−) (−) | (−) |
| 2 | 12.5 25 50 100 | 98.0 ± 2.2 91.0 ± 3.6 82.9 ± 1.2 65.7 ± 8.6 ** | 34.3 ± 4.7 35.1 ± 1.2 22.7 ± 0.5 ** (−) | 24.5 ± 10.2 22.7 ± 2.6 50.0 ± 1.0 (−) | 50.0 ± 0.3 |
| 3 | 12.5 25 50 100 | 93.5 ± 1.8 92.6 ± 3.1 88.7 ± 1.8 81.4 ± 2.3 | 38.5 ± 2.3 39.5 ± 0.6 35.6 ± 0.9 * 22.6 ± 0.4 ** | 15.2 ± 5.0 13.1 ± 1.2 21.6 ± 2.0 50.1 ± 0.9 | 99.8 ± 0.4 |
| 4 | 12.5 25 50 100 | 82.8 ± 2.7 80.7 ± 3.8 65.3 ± 2.3 ** 55.8 ± 2.7 ** | 38.4 ± 0.7 38.6 ± 3.3 (−) (−) | 15.5 ± 1.5 15.1 ± 7.3 (−) (−) | (−) |
| 5 | 12.5 25 50 100 | 98.9 ± 5.2 96.5 ± 9.2 95.9 ± 8.6 80.2 ± 5.7 | 38.3 ± 1.3 33.1 ± 1.0 * 22.1 ± 1.5 ** 14.8 ± 0.7 *** | 15.5 ± 2.8 27.1 ± 2.3 51.4 ± 3.2 67.3 ± 1.5 | 48.6 ± 1.2 |
| 6 | 12.5 25 50 100 | 94.8 ± 3.1 83.7 ± 3.5 83.5 ± 3.0 81.3 ± 2.8 | 37.0 ± 1.8 33.1 ± 2.4 * 22.9 ± 1.1 ** 15.1 ± 0.3 *** | 18.5 ± 4.0 27.1 ± 5.3 49.5 ± 2.4 66.7 ± 0.6 | 51.5 ± 0.5 |
| 7 | 12.5 25 50 100 | 98.6 ± 3.1 93.6 ± 4.0 92.5 ± 2.8 83.7 ± 1.6 | 42.6 ± 0.7 40.6 ± 2.8 23.7 ± 0.2 ** 14.3 ± 0.3 *** | 6.2 ± 1.5 10.6 ± 6.1 47.7 ± 0.3 68.5 ± 0.7 | 55.5 ± 0.3 |
| 8 | 12.5 25 50 100 | 98.4 ± 3.2 94.0 ± 2.6 88.6 ± 2.6 82.4 ± 1.2 | 39.8 ± 1.2 35.4 ± 0.8 * 23.5 ± 0.9 ** 14.4 ± 0.3 *** | 12.3 ± 2.6 21.9 ± 1.8 48.2 ± 2.0 68.3 ± 0.7 | 54.5 ± 0.8 |
| 9 | 12.5 25 50 100 | 99.9 ± 4.8 90.0 ± 1.6 82.6 ± 2.7 76.2 ± 1.1 * | 42.0 ± 1.2 40.4 ± 0.7 25.9 ± 2.3 (−) | 7.5 ± 2.7 11.0 ± 1.6 42.9 ± 5.0 (−) | (−) |
3. Experimental
3.1. General Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Determination of Inhibitory Effects on NO Production
3.5.1. Cell Culture
3.5.2. Cell Viability
3.5.3. Measurement of Nitric oxide/Nitrite
3.5.4. Statistical Analysis
4. Conclusion
Acknowledgments
References
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; International Book Distributors: Deharadun, India, 1987; pp. 68–69. [Google Scholar]
- Anonymous. The Useful Plants of India; Council of Scientific and Industrial Research: New Delhi, India, 1994; p. 43. [Google Scholar]
- Chang, F.R.; Chen, J.L.; Chiu, H.F.; Wu, M.J.; Wu, Y.C. Acetogenins from seeds of Annona reticulata. Phytochemistry 1998, 47, 1057–1061. [Google Scholar]
- Maeda, U.; Hara, N.; Fujimoto, Y.; Shrivastava, A.; Gupta, Y.K.; Sahai, M. N-fatty acyl tryptamines from Annona reticulata. Phytochemistry 1993, 34, 1633–1635. [Google Scholar] [CrossRef]
- Hisham, A.; Sunitha, C.; Sreekala, U.; Pieters, L.; De Bruyne, T.; Van den Heuvel, H.; Claeys, M. Reticulacinone, an acetogenin from Annona reticulata. Phytochemistry 1994, 35, 1325–1329. [Google Scholar] [CrossRef]
- Etse, J.T.; Waterman, P.G. Chemistry in the Annonaceae, XXII. 14-Hydroxy-25-desoxyrollinicin from the stem bark of Annona reticulata. J. Nat. Prod. 1986, 49, 684–686. [Google Scholar] [CrossRef]
- Saad, J.M.; Hui, Y.H.; Rupprecht, J.K.; Anderson, J.E.; Kozlowski, J.F.; Zhao, G.X.; Wood, K.V.; McLaughlin, J.L. Reticulatacin: A new bioactive acetogenin from Annona reticulata (Annonaceae). Tetrahedron 1991, 47, 2751–2756. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Saidutty, A. Analysis of the essential oils of the leaves and roots of Annona reticulata from South-India. Ernaehrung 1998, 22, 9–10. [Google Scholar]
- Hsieh, T.J.; Wu, Y.C.; Chen, S.C.; Huang, C.S.; Chen, C.Y. Chemical constituents from Annona glabra. J. Chin. Chem. Soc. 2004, 51, 869–876. [Google Scholar]
- Kanlayavattanakul, M.; Ruangrungsi, N.; Watanabe, T.; Kawahata, M.; Therrien, B.; Yamaguchi, K.; Ishikawa, T. ent-Halimane diterpenes and a guaiane sesquiterpene from Cladogynos orientalis. J. Nat. Prod. 2005, 68, 7–10. [Google Scholar] [CrossRef]
- Nes, W.D.; Norton, R.A.; Benson, M. Carbon-13-NMR studies on sitosterol biosynthesized from [13C]mevalonates. Phytochemistry 1992, 31, 805–811. [Google Scholar]
- Kuo, Y.H.; Chu, P.H. Studies on the constituents from the bark of Bauhinia purpurea. J. Chin. Chem. Soc. 2002, 49, 269–274. [Google Scholar]
- Chen, C.Y.; Chang, F.R.; Wu, Y.C. The constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1997, 44, 313–320. [Google Scholar]
- Yoshida, T.; Feng, W.S.; Okuda, T. Two polyphenol glycosides and tannins from Rosa cymosa. Phytochemistry 1993, 32, 1033–1036. [Google Scholar] [CrossRef]
- Srivastava, A.; Shukla, Y.N. Aryl esters and a coumarin from Aygyreia speciosa. Indian J. Chem. Sect. B 1998, 37B, 192–194. [Google Scholar]
- Okoye, T.C.; Akah, P.A.; Okoli, C.O.; Ezike, A.C.; Omeje, E.O.; Odoh, U.E. Antimicrobial effects of a lipophilic fraction and kaurenoic acid isolated from the root bark extracts of Annona senegalensis. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Guillopé, R.; Escobar-Khondiker, M.; Guérineau, V.; Laprévote, O.; Höglinger, G.U.; Champy, P. Kaurenoic acid from pulp of Annona cherimolia in regard to Annonaceae-induced Parkinsonism. Phytother. Res. 2011, 25, 1861–1864. [Google Scholar] [CrossRef]
- Choi, R.J.; Shin, E.M.; Jung, H.A.; Choi, J.S.; Kim, Y.S. Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages. Phytomedicine 2011, 18, 677–682. [Google Scholar] [CrossRef]
- Mizokami, S.S.; Arakawa, N.S.; Ambrosio, S.R.; Zarpelon, A.C.; Casagrande, R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Kaurenoic acid from Sphagneticola trilobata inhibits inflammatory pain: Effect on cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway. J. Nat. Prod. 2012, 75, 896–904. [Google Scholar] [CrossRef]
- Lim, H.; Jung, H.A.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the constituents of the roots of Aralia continentalis. Arch. Pharm. Res. 2009, 32, 1237–1243. [Google Scholar] [CrossRef]
- Batista, R.; García, P.A.; Castro, M.A.; Miguel Del Corral, J.M.; Speziali, N.L.; de P Varotti, F.; de Paula, R.C.; García-Fernández, L.F.; Francesch, A.; San Feliciano, A.; et al. Synthesis, cytotoxicity and antiplasmodial activity of novel ent-kaurane derivatives. Eur. J. Med. Chem. 2013, 62C, 168–176. [Google Scholar]
- De Andrade, B.B.; Moreira, M.R.; Ambrosio, S.R.; Furtado, N.A.; Cunha, W.R.; Heleno, V.C.; Silva, A.N.; Simão, M.R.; Da Rocha, E.M.; Martins, C.H.; et al. Evaluation of ent-kaurenoic acid derivatives for their anticariogenic activity. Nat. Prod. Commun. 2011, 6, 777–780. [Google Scholar]
- Raga, D.D.; Alimboyoguen, A.B.; Del Fierro, R.S.; Ragasa, C.Y. Hypoglycaemic effects of tea extracts and ent-kaurenoic acid from Smallanthus sonchifolius. Nat. Prod. Res. 2010, 24, 1771–1782. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; da Costa, F.B.; Coutinho, S.T.; de Oliveira, D.C.; de Oliveira, A.M. Analysis of the mechanisms underlying the vasorelaxant action of kaurenoic acid in the isolated rat aorta. Eur. J. Pharmacol. 2004, 492, 233–241. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Tirapelli, C.R.; Coutinho, S.T.; de Oliveira, D.C.; de Oliveira, A.M.; da Costa, F.B. Role of the carboxylic group in the antispasmodic and vasorelaxant action displayed by kaurenoic acid. J. Pharm. Pharmacol. 2004, 56, 1407–1413. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chang, F.R.; Wu, C.C.; Wang, W.Y.; Wu, Y.C. New ent-kaurane diterpenoids with anti-platelet aggregation activity from Annona squamosa. J. Nat. Prod. 2002, 65, 1462–1467. [Google Scholar] [CrossRef]
- Yao, X.; Li, G.; Bai, Q.; Xu, H.; Lü, C. Taraxerol inhibits LPS-induced inflammatory responses through suppression of TAK1 and Akt activation. Int.Immunopharmacol. 2013, 15, 316–324. [Google Scholar] [CrossRef]
- Chang, C.T.; Huang, S.S.; Lin, S.S.; Amagaya, S.; Ho, H.Y.; Hou, W.C.; Shie, P.H.; Wu, J.B.; Huang, G.J. Anti-inflammatory activities of tormentic acid from suspension cells of Eriobotrya japonica ex vivo and in vivo. Food Chem. 2011, 127, 1131–1137. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–9 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thang, T.D.; Kuo, P.-C.; Huang, G.-J.; Hung, N.H.; Huang, B.-S.; Yang, M.-L.; Luong, N.X.; Wu, T.-S. Chemical Constituents from the Leaves of Annona reticulata and Their Inhibitory Effects on NO Production. Molecules 2013, 18, 4477-4486. https://doi.org/10.3390/molecules18044477
Thang TD, Kuo P-C, Huang G-J, Hung NH, Huang B-S, Yang M-L, Luong NX, Wu T-S. Chemical Constituents from the Leaves of Annona reticulata and Their Inhibitory Effects on NO Production. Molecules. 2013; 18(4):4477-4486. https://doi.org/10.3390/molecules18044477
Chicago/Turabian StyleThang, Tran Dinh, Ping-Chung Kuo, Guan-Jhong Huang, Nguyen Huy Hung, Bow-Shin Huang, Mei-Lin Yang, Ngo Xuan Luong, and Tian-Shung Wu. 2013. "Chemical Constituents from the Leaves of Annona reticulata and Their Inhibitory Effects on NO Production" Molecules 18, no. 4: 4477-4486. https://doi.org/10.3390/molecules18044477




