Synthesis of New 1,2,3-Triazole Derivatives of Uracil and Thymine with Potential Inhibitory Activity against Acidic Corrosion of Steels
Abstract
:1. Introduction
2. Results and Discussion
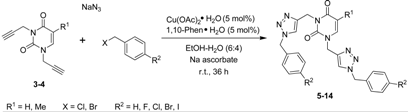
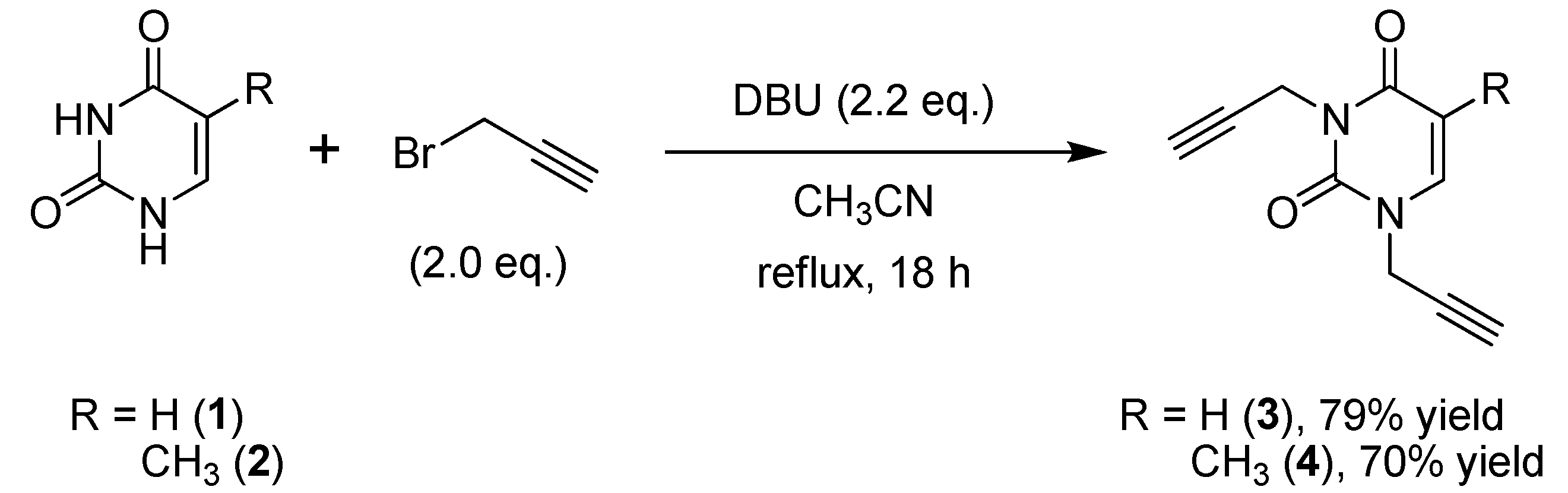
2.1. Synthesis
| Entry | Compound | R1 | R2 | X | Yield a (%) |
|---|---|---|---|---|---|
| 1 b | 5 | H | H | Cl | 44 |
| 2 | 5 | H | H | Cl | 74 |
| 3 | 6 | H | F | Cl | 78 |
| 4 | 7 | H | Cl | Cl | 71 |
| 5 | 8 | H | Br | Br | 87 |
| 6 | 9 | H | I | Br | 84 |
| 7 | 10 | CH3 | H | Cl | 64 |
| 8 | 11 | CH3 | F | Cl | 87 |
| 9 | 12 | CH3 | Cl | Cl | 74 |
| 10 | 13 | CH3 | Br | Br | 73 |
| 11 | 14 | CH3 | I | Br | 86 |
| Compound | R1 | R2 | H-5 | C-5 | C-4 |
|---|---|---|---|---|---|
| 5 | H | H | 7.49/7.62 | 123.4/123.7 | 142.3/143.4 |
| 6 | H | F | 7.96/8.12 | 124.0/124.3 | 143.0/143.4 |
| 7 | H | Cl | 7.98/8.14 | 124.2/124.5 | 143.0/143.4 |
| 8 | H | Br | 7.98/8.13 | 124.2/124.5 | 143.0/143.4 |
| 9 | H | I | 7.95/8.12 | 124.1/124.5 | 143.0/143.4 |
| 10 | CH3 | H | 7.49/7.62 | 123.5/123.7 | 142.6/143.5 |
| 11 | CH3 | F | 7.96/8.12 | 124.0/124.3 | 143.1/143.5 |
| 12 | CH3 | Cl | 7.50/7.64 | 123.5/123.7 | 142.8/143.7 |
| 13 | CH3 | Br | 7.50/7.65 | 123.5/123.7 | 142.8/143.7 |
| 14 | CH3 | I | 7.95/8.11 | 124.1/124.4 | 143.1/143.5 |
2.2. Corrosion Inhibition Efficiencies
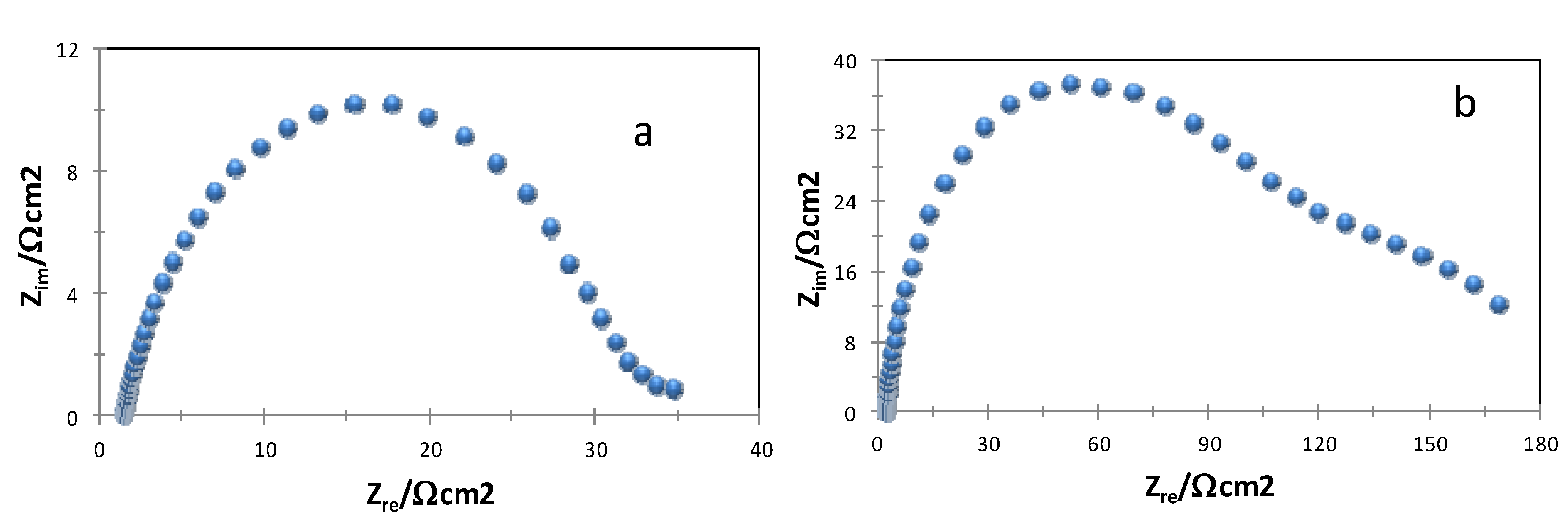
| Compound | Rs/Ω cm2 | Rp/Ω cm2 | C/µF | IE/% |
|---|---|---|---|---|
| Blank | 2.4 | 30 | 310 | - |
| 1 | 2.5 | 242 | 94 | 88 |
| 2 | 7.1 | 304 | 97 | 90 |
| 3 | 2.0 | 163 | 167 | 82 |
| 4 | 1.8 | 233 | 100 | 87 |
3. Experimental
3.1. General
3.2. Product Synthesis and Characterization
4. Conclusions
Acknowledgments
References
- Dinakaran, V.S.; Bhargavi, B.; Srinivasan, K.K. Fused pyrimidines: The heterocycle of diverse biological and pharmacological significance. Der Pharma Chem. 2012, 4, 255–265. [Google Scholar]
- Sugimachi, K.; Maehara, Y.; Ogawa, M.; Kakegawa, T.; Tomita, M. Dose intensity of uracil and tegafur in postoperative chemotherapy for patients with poorly differentiated gastric cancer. Cancer Chemother. Pharmacol. 1997, 40, 233–238. [Google Scholar] [CrossRef]
- Kurebayashi, J.; Nukatsuka, M.; Fujoka, A.; Saito, H.; Takeda, S.; Unemi, N.; Fukumori, H.; Kurosumi, M.; Sonoo, H.; Dickson, R.B. Postsurgical oral adminitratios of uracil and tegafur inhibts progession of micrometastasis of human breast cancer cells in nude mice. Clin. Cancer Res. 1997, 3, 653–659. [Google Scholar]
- Mani, S.; Sciortino, D.; Samuels, B.; Arrietta, R.; Schilsky, R.L.; Vokes, E.E.; Benner, S. Phase II trial of uracil/tegafur (UFT) plus leucovorin in patients with advanced biliary carcinoma. Invest. New Drugs 1999, 17, 97–101. [Google Scholar]
- Yadav, D.K.; Quraishi, M.A. Application of Some Condensed Uracils as Corrosion Inhibitors for Mild Steel: Gravimetric, Electrochemical, Surface Morphological, UV-Visible, and Theoretical Investigations. Ind. Eng. Chem. Res. 2012, 51, 14966–14979. [Google Scholar] [CrossRef]
- Loto, R.T.; Loto, C.A.; Popoola, A.P.I.; Ranyaoa, M. Pyrimidine derivatives as environmentally-friendly corrosion inhibitors: A review. Int. J. Phys. Sci. 2012, 7, 2697–2705. [Google Scholar]
- Udhayakala, P.; Rajendiran, T.V.; Gunasekaran, S. Theoretical approach to the corrosion inhibition efficiency of some pyrimidine derivatives using DFT method. J. Comput. Meth. Mol. Des. 2012, 2, 1–15. [Google Scholar]
- Bosch, L.; Delelis, O.; Subra, F.; Deprez, E.; Witvrow, M.; Vilarrasa, J. Thymidine- and AZT-linked 5-(1,3-dioxoalkyl)tetrazoles and 4-(1,3-dioxoalkyl)-1,2,3-triazoles. Tetrahedron Lett. 2012, 53, 514–518. [Google Scholar] [CrossRef]
- Abiola, O.K.; John, M.O.; Asekunowo, P.O.; Okafor, P.C.; James, O.O. 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methyl thiazolium chloride hydrochloride as green corrosion inhibitor of copper in HNO3 solution and its adsorption characteristics. Green Chem. Lett. Rev. 2011, 4, 273–279. [Google Scholar] [CrossRef]
- Khaled, K.F.; Hamed, M.N.H.; Abdel-Azim, K.M.; Abdelshafi, N.S. Inhibition of copper corrosion in 3.5% NaCl solutions by a new pyrimidine derivative: Electrochemical and computer simulation techniques. J. Solid State Electrochem. 2011, 15, 663–673. [Google Scholar] [CrossRef]
- Caliskan, N.; Akbas, E. The inhibition effect of some pyrimidine derivatives on austenitic stainless steel in acidic media. Mater. Chem. Phys. 2011, 126, 983–988. [Google Scholar] [CrossRef]
- Masoud, M.S.; Awad, M.K.; Shaker, M.A.; El-Tahawy, M.M.T. The role of structural chemistry in the inhibitive performance of some aminopyrimidines on the corrosion of steel. Corros. Sci. 2010, 52, 2387–2396. [Google Scholar] [CrossRef]
- Sathya, P.; Parameswari, K.; Chitra, S.; Selvaraj, A. A comparative study of the corrosion inhibitive properties of pyrimidine derivatives for mild steel. E-J. Chem. 2009, 6, S65–S74. [Google Scholar] [CrossRef]
- Elewady, G.Y. Pyrimidine Derivatives as Corrosion Inhibitors for Carbon-Steel in 2M Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2008, 3, 1149–1161. [Google Scholar]
- Issa, R.M.; Awad, M.K.; Atlam, F.M. Quantum chemical studies on the inhibition of corrosion of copper surface by substituted uracils. Appl. Surf. Sci. 2008, 255, 2433–2441. [Google Scholar] [CrossRef]
- Fouda, A.S.; El-Dafrawy, H. Inhibitive effect of some pyrimidine derivatives on the cyclic stressed specimens of stainless steel type 304 in acidic media. Int. J. Electrochem. Sci. 2007, 2, 721–733. [Google Scholar]
- Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Angeles-Beltrán, D.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Herrera-Hernández, H. Electrochemical impedance evaluation of uracil and thymine pyrimidine derivatives and its nucleosides compounds as a non-toxic corrosion inhibitors of steels in 1M HCl. ECS Trans. 2011, 36, 217–228. [Google Scholar]
- Dabak, K.; Sezer, O.; Akar, A.; Anac, O. Synthesis and investigation of tuberculosis inhibition activities of some 1,2,3-triazole derivatives. Eur. J. Med. Chem. 2003, 38, 215–218. [Google Scholar] [CrossRef]
- Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Efficient synthesis and in vitro antitubercular activity of 1,2,3-triazoles as inhibitors of Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2011, 21, 7273–7276. [Google Scholar] [CrossRef]
- Kai, H.; Hinou, H.; Nishimura, S.-I. Aglycone-focused randomization of 2-difluoromethylphenyltypesialoside suicide substrates for neuraminidases. Bioorg. Med. Chem. 2012, 20, 2739–2746. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P.; Anand, A.; Bedi, P.M.S.; Kaur, T.; Saxena, A.K.; Kumar, V. Azide-alkyne cycloaddition en route to novel 1H-1,2,3-triazole tethered isatin conjugates with in vitro cytotoxic evaluation. Eur. J. Med. Chem. 2012, 55, 455–461. [Google Scholar] [CrossRef]
- Lakshman, M.K.; Kumar, A.; Balachandran, R.; Day, B.W.; Andrei, G.; Snoeck, R.; Balzarini, J. synthesis and biological properties of C-2 triazolylinosine derivatives. J. Org. Chem. 2012, 77, 5870–5883. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, Q.; Hu, H.; Hu, L.; Yu, S.; Xu, M.; Wu, Q. Synthesis and in vitro antitumor activities of xanthone derivatives containing 1,4-disubstituted-1,2,3-triazole moiety. Arch. Pharm. Res. 2012, 35, 2093–2104. [Google Scholar] [CrossRef]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.S.; Kaur, T.; Saxena, A.K. 1,2,3-Triazole tethered β-lactam-Chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar] [CrossRef]
- Al-Masoudi, N.A.; Pfleiderer, W.; Pannecouque, C. Nitroimidazoles part 7. Synthesis and anti-HIV activity of new 4-nitroimidazole derivative. J. Chem. Sci. 2012, 67, 835–842. [Google Scholar]
- Kanishchev, O.S.; Gudz, G.P.; Shermolovich, Y.G.; Nesterova, N.V.; Zagorodnya, S.D.; Golovan, A.V. Synthesis and biological activity of the nucleoside analogs based on polyfluoroalkyl-substituted 1,2,3-triazoles. Nucleo. Nucleot. Nucleic Acids 2011, 30, 768–783. [Google Scholar] [CrossRef]
- Cheng, H.; Wan, J.; Lin, M.I.; Liu, Y.; Lu, X.; Liu, J.; Xu, Y.; Chen, J.; Tu, Z.; Cheng, Y.S.E.; Ding, K. Design, synthesis, and in vitro biological evaluation of 1H-1,2,3-Triazole-4-carboxamide derivatives as new anti-influenza A agents targeting virus nucleoprotein. J. Med. Chem. 2012, 55, 2144–2153. [Google Scholar] [CrossRef]
- Turan-Zitouni, G.; Kaplancikli, Z.A.; Erol, K.; Kilic, F.S. Synthesis and analgesic activity of some triazoles and triazolo-thiadiazines. Farmaco 1999, 54, 218–223. [Google Scholar] [CrossRef]
- He, J.; Feng, L.; Li, J.; Tao, R.; Wang, F.; Liao, X.; Sun, Q.; Long, Q.; Ren, Y.; Wan, J.; He, H. Design, synthesis and biological evaluation of novel 2-methylpyrimidine-4-ylamine derivatives as inhibitors of Escherichia coli pyruvate dehydrogenase complex E1. Bioorg. Med. Chem. 2012, 20, 1665–1670. [Google Scholar] [CrossRef]
- Lima-Neto, R.G.; Cavalcante, N.N.M.; Srivastava, R.M.; Mendonca, F.J.B., Jr.; Wanderley, A.G.; Neves, R.P.; dos Anjos, J.V. Synthesis of 1,2,3-triazole derivatives and in vitro antifungal evaluation on Candida strains. Molecules 2012, 17, 5882–5892. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, B.W.; Lu, J.R.; Xin, C.W.; Li, J.F.; Liu, Y. Design, synthesis and antimicrobial activities of 1,2,3-triazole derivatives. Chin. Chem. Lett. 2012, 23, 933–935. [Google Scholar] [CrossRef]
- He, X.-P.; Xie, J.; Tang, Y.; Li, J.; Chen, G.-R. CuAAC Click chemistry accelerates the discovery of novel chemical scaffolds as promising protein tyrosine phosphatases inhibitors. Curr. Med. Chem. 2012, 19, 2399–2405. [Google Scholar] [CrossRef]
- Luo, L.; He, X.-P.; Shen, Q.; Li, J.-Y.; Shi, X.-X.; Xie, J.; Li, J.; Chen, G.-R. Synthesis of (Glycopyrasonyl-tryazolyl-purines and their inhibitory Activities against Protein Tyrosine Phosphatase 1B (PTP1B). Chem. Biodivers. 2011, 8, 2035–2044. [Google Scholar] [CrossRef]
- Gramlich, P.M.E.; Wirges, C.T.; Manetto, A.; Carell, T. Postsynthetic DNA Modification through the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction. Angew. Chem. Int. Ed. 2008, 47, 8350–8358. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.-H.; Schinazi, R.F. Cu(I)-Catalyzed Huisgen Azide-Alkyne 1,3-Dipolar Cycloaddition Reaction in Nucleoside, Nucleotide, and Oligonucleotide Chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Qu, Y.; Song, Z.; He, X.-P.; Xie, J.; Hua, J.; Chen, G.-R. Discovery of a sensitive Cu(II)-cyanide “off-on” sensor based on new C-glycosyl triazolyl bis-amino acid scaffold. Org. Biomol. Chem. 2012, 10, 555–560. [Google Scholar] [CrossRef]
- Beckendorf, S.; Asmus, S.; Mück-Lichtenfeld, C.; García Mancheño, O. "Click” Bis-Triazoles as Neutral C-H⋅Anion-Acceptor Organocatalysts. Chem. Eur. J. 2013, 19, 1581–1585. [Google Scholar] [CrossRef]
- Hamdy, H.H.; Essam, A.; Mohammed, A.A. Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives Part I. Polarization and EIS studies. Electrochim. Acta 2007, 52, 6359–6366. [Google Scholar] [CrossRef]
- Li, W.; He, Q.; Pei, C.; Hou, B. Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim. Acta 2007, 52, 6386–6394. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. Effects of 3-amino-1,2,4-triazole on the inhibition of copper corrosion in acidic chloride solutions. J. Colloid Interface Sci. 2007, 311, 144–151. [Google Scholar] [CrossRef]
- Bentiss, F.; Bouanis, M.; Mernari, B.; Traisnel, M.; Vezin, H.; Lagrenée, M. Understanding the adsorption of 4H-1,2,4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl. Surf. Sci. 2007, 253, 3696–3704. [Google Scholar] [CrossRef]
- Allam, N.K. Thermodynamic and quantum chemistry characterization of the adsorption of triazole derivatives during Muntz corrosion in acidic and neutral solutions. Appl. Surf. Sci. 2007, 253, 4570–4577. [Google Scholar] [CrossRef]
- Lebrini, M.; Traisnel, M.; Lagrenée, M.; Mernari, B.; Bentiss, F. Inhibitive properties, adsorption and a theoretical study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild steel in perchloric acid. Corros. Sci. 2008, 50, 473–479. [Google Scholar] [CrossRef]
- Xu, F.; Duan, J.; Zhang, S.; Hou, B. The inhibition of mild steel corrosion in 1 M hydrochloric acid solutions by triazole derivative. Mater. Lett. 2008, 62, 4072–4074. [Google Scholar] [CrossRef]
- Khaled, K.F. Molecular simulation, quantum chemical calculations and electrochemical studies for inhibition of mild steel by triazoles. Electrochim. Acta 2008, 53, 3484–3492. [Google Scholar] [CrossRef]
- Qiao, W.L.; Zhang, H.S.; Pei, C.; Hou, B. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J. Appl. Electrochem. 2008, 38, 289–295. [Google Scholar] [CrossRef]
- Tang, Y.M.; Chen, Y.; Yang, W.Z.; Liu, Y.; Yin, X.S.; Wang, J.T. Electrochemical and theoretical studies of thienyl-substituted amino triazoles on corrosion inhibition of copper in 0.5 M H2SO4. J. Appl. Electrochem. 2008, 38, 1553–1559. [Google Scholar] [CrossRef]
- Bentiss, F.; Jama, C.; Mernari, B.; Attari, H.E.; Kadi, L.E.; Lebrini, M.; Traisnel, M.; Lagrenée, M. Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4- triazole in normal hydrochloric acid medium. Corros. Sci. 2009, 51, 1628–1635. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, S.; Li, W.; Hou, B. Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros. Sci. 2009, 51, 2588–2595. [Google Scholar] [CrossRef]
- Khadom, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S. Adsorption kinetics of 4-amino-5-phenyl-4h-1,2,4-triazole-3-thiol on mild steel surface. Portugaliae Electrochim. Acta 2010, 28, 221–230. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Daud, A.R. Siti kartom kamarudin on the inhibition of mild steel corrosion by 4-amino-5-phenyl-4h-1,2,4-trizole-3-thiol. Corros. Sci. 2010, 52, 526–533. [Google Scholar] [CrossRef]
- Song, S.-X.; Zhang, H.-L.; Kim, C.-G.; Sheng, L.; He, X.-P.; Long, Y.-T.; Li, J.; Chen, G.-R. Expeditious preparation of triazole-linked glycolipids via microwave accelerated click chemistry and their electrochemical and biological assessments. Tetrahedron 2010, 66, 9974–9980. [Google Scholar] [CrossRef]
- Mert, B.D.; Mert, M.E.; Kardas, G.; Yazici, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]
- John, S.; Joseph, A. Electro analytical, surface morphological and theoretical studies on the corrosion inhibition behavior of different 1,2,4-triazole precursors on mild steel in 1 M hydrochloric acid. Mater. Chem. Phys. 2012, 133, 1083–1091. [Google Scholar] [CrossRef]
- Deng, Q.; He, X.-P.; Shi, H.-W.; Chen, B.-Q.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R.; Chen, K. Concise Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction ligation remarkably enhances the corrosion inhibitive potency of natural amino acids for mild steel in HCl. Ind. Eng. Chem. Res. 2012, 51, 7160–7169. [Google Scholar]
- Deng, Q.; Ding, N.-N.; Wei, X.-L.; Cai, L.; He, X.-P.; Long, Y.-T.; Chen, G.-R.; Chen, K. Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.-W.; Ding, N.-N.; Chen, B.-Q.; He, X.-P.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R. Novel triazolyl bis-amino acid derivatives readily synthesized via click chemistry as potential corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 57, 220–227. [Google Scholar] [CrossRef]
- Appkkuttan, P.; Dehaen, W.; Fokin, V.V.; van der Eycken, E. A Microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper(I)-catalyzed three-component reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef]
- Albadi, J.; Keshavarz, M.; Shirini, F.; Vafaie-nezhad, M. Copper iodide nanoparticles on poly(4-vinylpyridine): A new and efficient catalyst for multicomponent click synthesis of 1,4-disubstituted-1,2,3-triazoles in water. Catal. Commun. 2012, 27, 17–20. [Google Scholar]
- Jin, G.; Zhang, J.; Fu, D.; Wu, J.; Cao, S. One-Pot, Three-component synthesis of 1,4,5-trisubstituted 1,2,3-triazoles starting from primary alcohols. Eur. J. Org. Chem. 2012, 5446–5449. [Google Scholar]
- Hosseinzadeh, R.; Sepehrian, H.; Shahrokhi, F. Preparation of Cu(OAc)2/MCM-41 catalyst and its application in the one-pot synthesis of 1,2,3-triazoles in water. Heteroatom Chem. 2012, 23, 415–421. [Google Scholar] [CrossRef]
- Kumar, B.S.P.A.; Reddy, K.H.V.; Madhav, B.; Ramesh, K.; Nageswar, Y.V.D. Magnetically separable CuFe2O4 nano particles catalyzed multicomponent synthesis of 1,4-disubstituted 1,2,3-triazoles in tap water using 'click chemistry'. Tetrahedron Lett. 2012, 53, 4595–4599. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Multicomponent click synthesis of 1,2,3-triazoles from epoxides in water catalyzed by copper nanoparticles on activated carbon. J. Org. Chem. 2011, 76, 8394–8405. [Google Scholar] [CrossRef] [Green Version]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, G.M.; Anjum, S.R. Cu(OTf)2/Cu-catalyzed four-component reaction: a facile synthesis of α-alkoxytriazoles via click chemistry. Tetrahedron Lett. 2009, 50, 6029–6031. [Google Scholar] [CrossRef]
- Kalisiak, J.; Sharpless, K.B.; Fokin, V.V. Efficient synthesis of 2-substituted-1,2,3-triazoles. Org. Lett. 2008, 10, 3171–3174. [Google Scholar] [CrossRef]
- Sreedhar, B.; Reddy, P.S.; Krishna, V.R. Regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles via three-component coupling of secondary alcohols, TMSN3 and alkynes. Tetrahedron Lett. 2007, 48, 5831–5834. [Google Scholar] [CrossRef]
- Hwang, S.; Bae, H.; Kim, S.; Kim, S. An efficient and high-yielding one-pot synthesis of 4-acyl-1,2,3-triazoles via triisopropylsilyl-protected ynones. Tetrahedron 2012, 68, 1460–1465. [Google Scholar] [CrossRef]
- Krim, J.; Taourirte, M.; Engels, J.W. Synthesis of 1,4-disubstituted mono and bis-triazolocarbo-acyclonucleoside analogues of 9-(4-hydroxybutyl)guanine by Cu(I)-catalyzed click azide-alkyne cycloaddition. Molecules 2012, 17, 179–190. [Google Scholar]
- Brotherton, W.S.; Clark, R.J.; Zhu, L. Synthesis of 5-iodo-1,4-disubstituted-1,2,3-triazoles mediated by in situ generated copper(I) catalyst and electrophilic triiodide ion. J. Org. Chem. 2012, 77, 6443–6455. [Google Scholar] [CrossRef]
- Stefani, H.A.; Amaral, M.F.Z.J.; Manarin, F.; Ando, R.A.; Silva, N.C.S.; Juaristi, E. Functionalization of 2-(S)-isopropyl-5-iodo-pyrimidin-4-ones through Cu(I)-mediated 1,3-dipolar azide-alkyne cycloadditions. Tetrahedron Lett. 2011, 52, 6883–6886. [Google Scholar] [CrossRef]
- Wang, F.; Fu, H.; Jiang, Y.; Zhao, Y. Quick and highly efficient copper-catalyzed cycloaddition of aliphatic and aryl azides with terminal alkynes “on water”. Green Chem. 2008, 10, 452–456. [Google Scholar] [CrossRef]
- Saraiva, M.T.; Seus, N.; de Souza, S.; Rodrigues, O.E.D.; Paixão, M.W.; Jacob, R.G.; Lenardão, E.J.; Perin, G.; Alves, D. Synthesis of [(arylselanyl)alkyl]-1,2,3-triazoles by copper-catalyzed 1,3-dipolar cycloaddition of (arylselanyl)alkynes with benzyl azides. Synthesis 2012, 44, 1997–2004. [Google Scholar] [CrossRef]
- Kuang, G.-C.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Zhu, L. Chelation-assisted, copper(II)-acetate-accelerated azide-alkyne cycloaddition. J. Org. Chem. 2010, 75, 6540–6548. [Google Scholar] [CrossRef]
- Brotherton, W.S.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Dalal, N.S.; Zhu, L. Apparent copper(ii)-accelerated azide-alkyne cycloaddition. Org. Lett. 2009, 11, 4954–4057. [Google Scholar] [CrossRef]
- Lewis, W.G.; Magallon, F.G.; Fokin, V.V.; Finn, M.G. Discovery and characterization of catalysts for azide-alkyne cycloaddition by fluorescence quenching. J. Am. Chem. Soc. 2004, 126, 9152–9153. [Google Scholar] [CrossRef]
- Creary, X.; Anderson, A.; Brophy, C.; Crowell, F.; Funk, Z. Method for assigning structure of 1,2,3-triazoles. J. Org. Chem. 2012, 77, 8756–8761. [Google Scholar] [CrossRef]
- Krim, J.; Sillahi, B.; Taourirte, M.; Rakib, E.M.; Engels, J.W. Microwave-assisted click chemistry: Synthesis of mono and bis-1,2,3-triazole acyclonucleoside analogues of ACV via copper(I)-catalyzed cycloaddition. Arkivoc 2009, xiii, 142–152. [Google Scholar]
- Sample Availability: Samples of the compounds 3–14 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Maldonado-Carmona, N.; Espinoza-Vázquez, A.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Synthesis of New 1,2,3-Triazole Derivatives of Uracil and Thymine with Potential Inhibitory Activity against Acidic Corrosion of Steels. Molecules 2013, 18, 4613-4627. https://doi.org/10.3390/molecules18044613
Negrón-Silva GE, González-Olvera R, Angeles-Beltrán D, Maldonado-Carmona N, Espinoza-Vázquez A, Palomar-Pardavé ME, Romero-Romo MA, Santillan R. Synthesis of New 1,2,3-Triazole Derivatives of Uracil and Thymine with Potential Inhibitory Activity against Acidic Corrosion of Steels. Molecules. 2013; 18(4):4613-4627. https://doi.org/10.3390/molecules18044613
Chicago/Turabian StyleNegrón-Silva, Guillermo E., Rodrigo González-Olvera, Deyanira Angeles-Beltrán, Nidia Maldonado-Carmona, Araceli Espinoza-Vázquez, Manuel E. Palomar-Pardavé, Mario A. Romero-Romo, and Rosa Santillan. 2013. "Synthesis of New 1,2,3-Triazole Derivatives of Uracil and Thymine with Potential Inhibitory Activity against Acidic Corrosion of Steels" Molecules 18, no. 4: 4613-4627. https://doi.org/10.3390/molecules18044613