A Series of Asymmetrical Phthalocyanines: Synthesis and Near Infrared Properties
Abstract
:1. Introduction
2. Results and Discussion
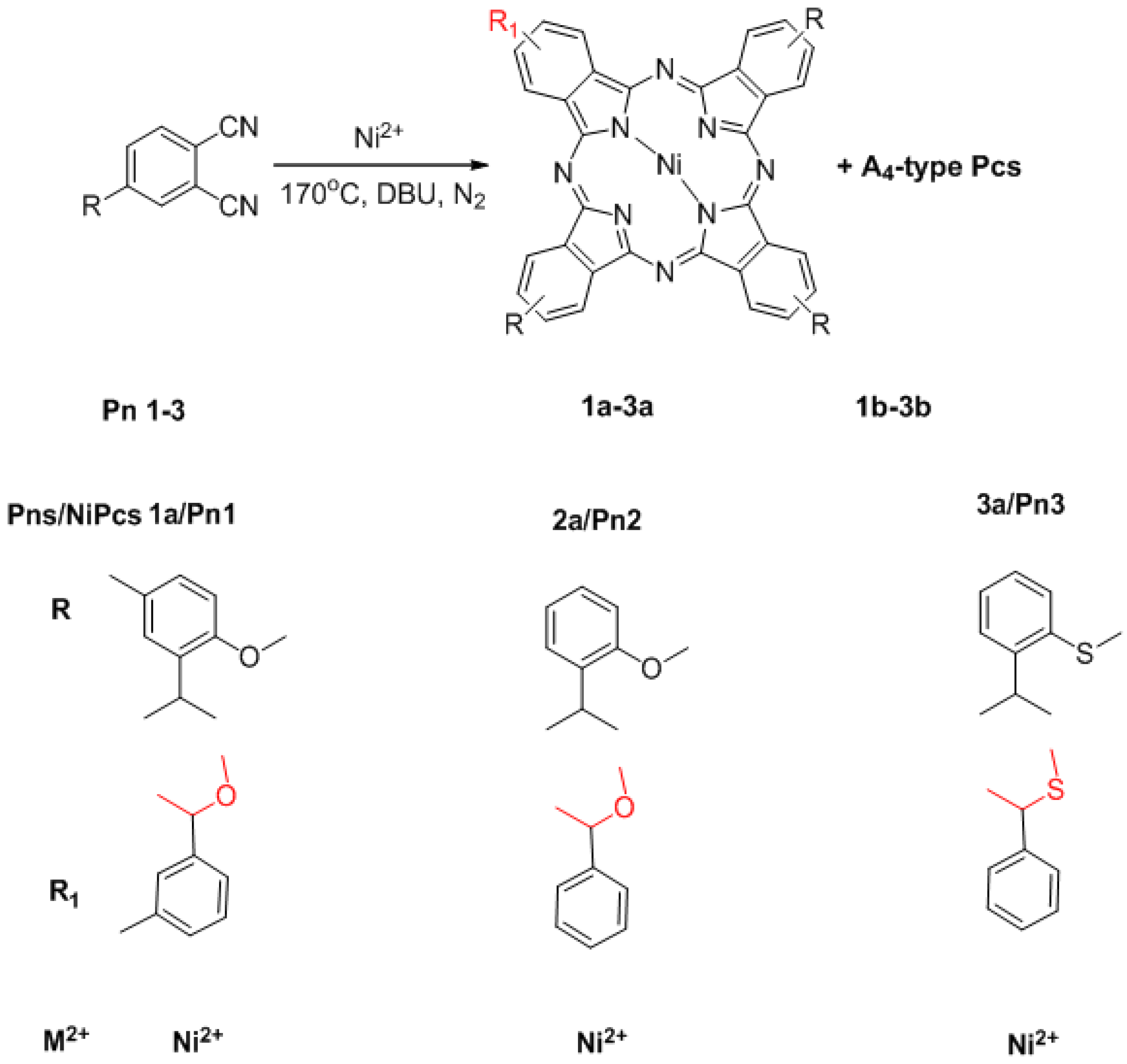
2.1. Synthesis
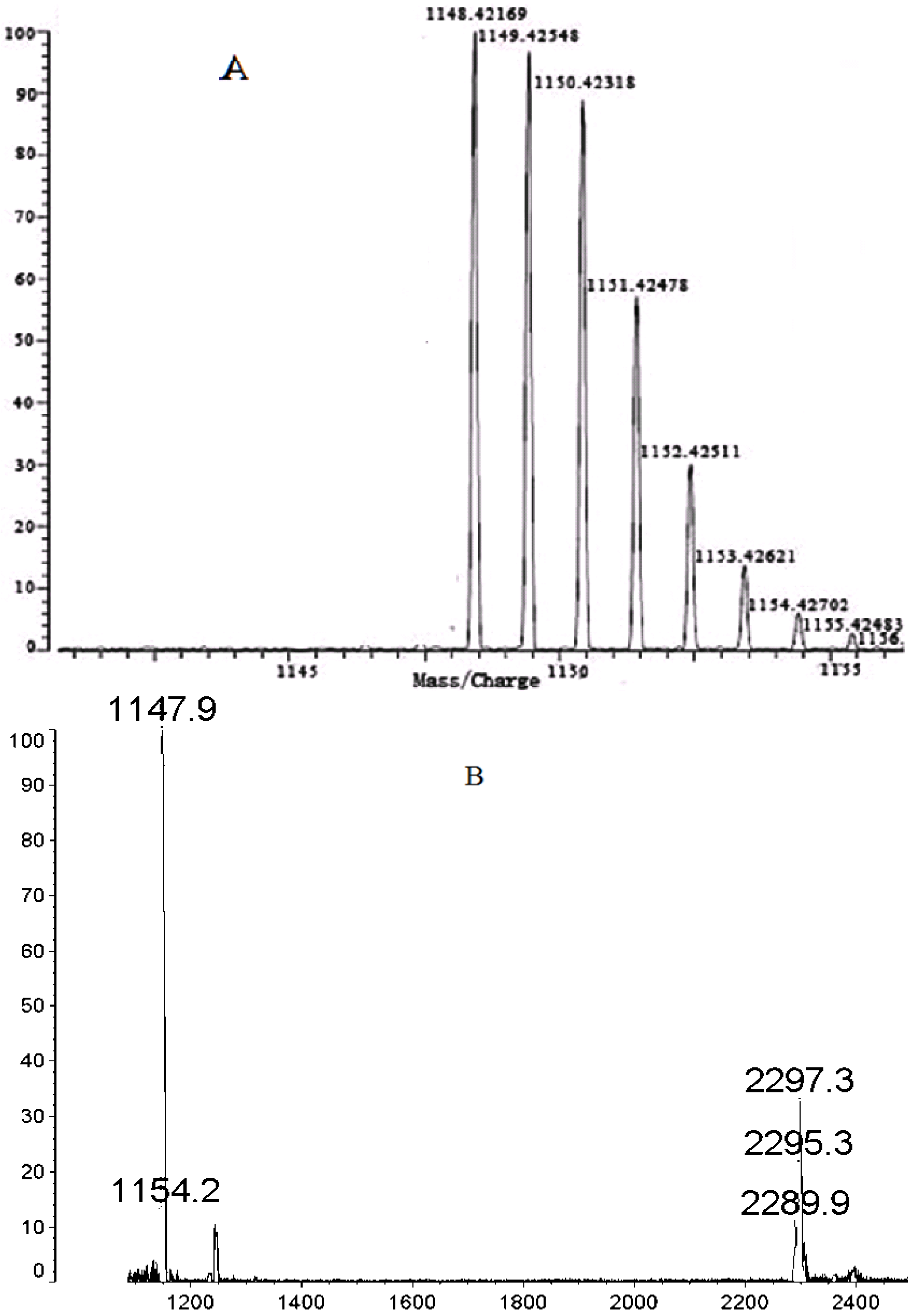
2.2. High Resolution MS
2.3. 1H-13C-NMR

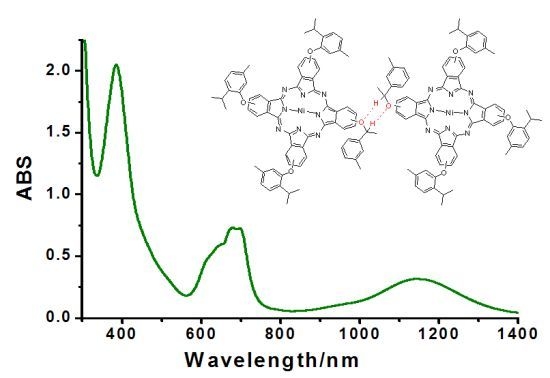
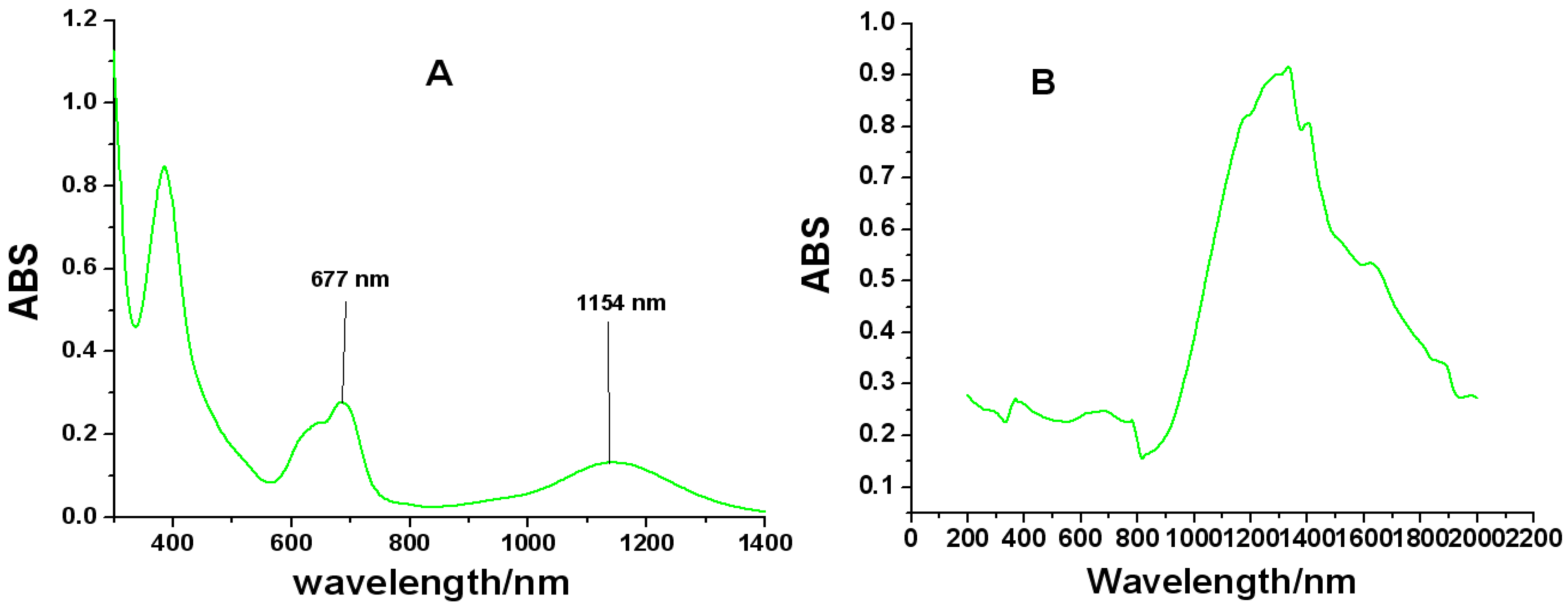
2.4. UV-Vis-NIR Spectroscopy
2.5. NIR Luminescence

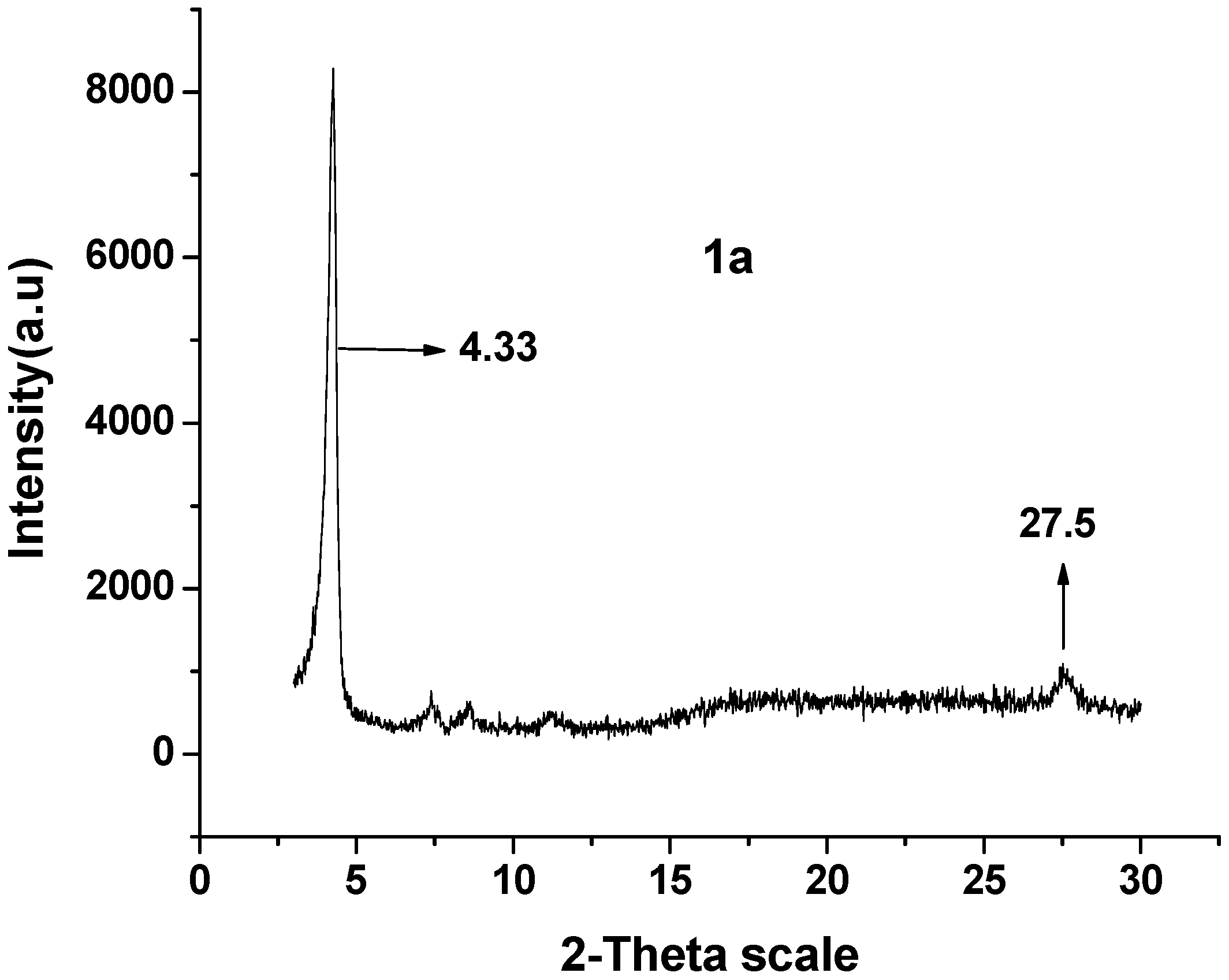
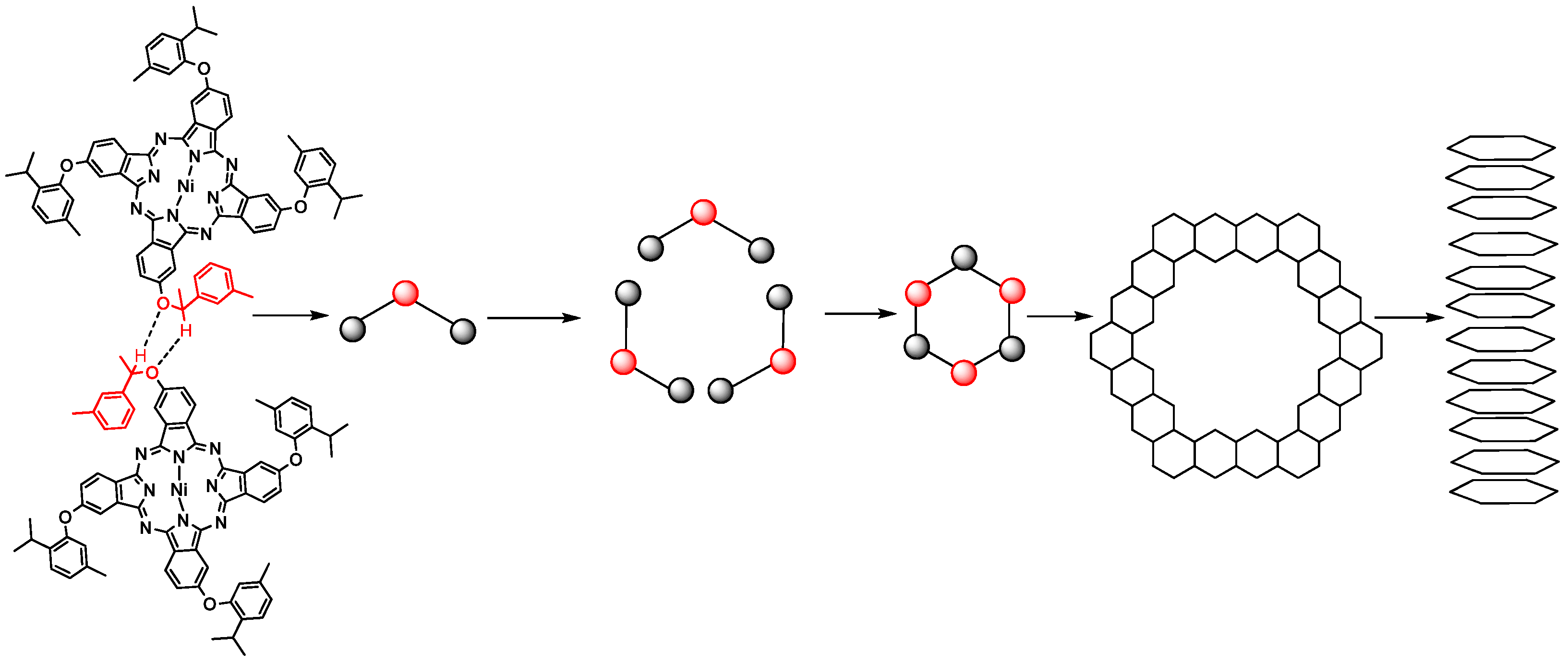
2.6. 1-D Molecular Morphology and XRD

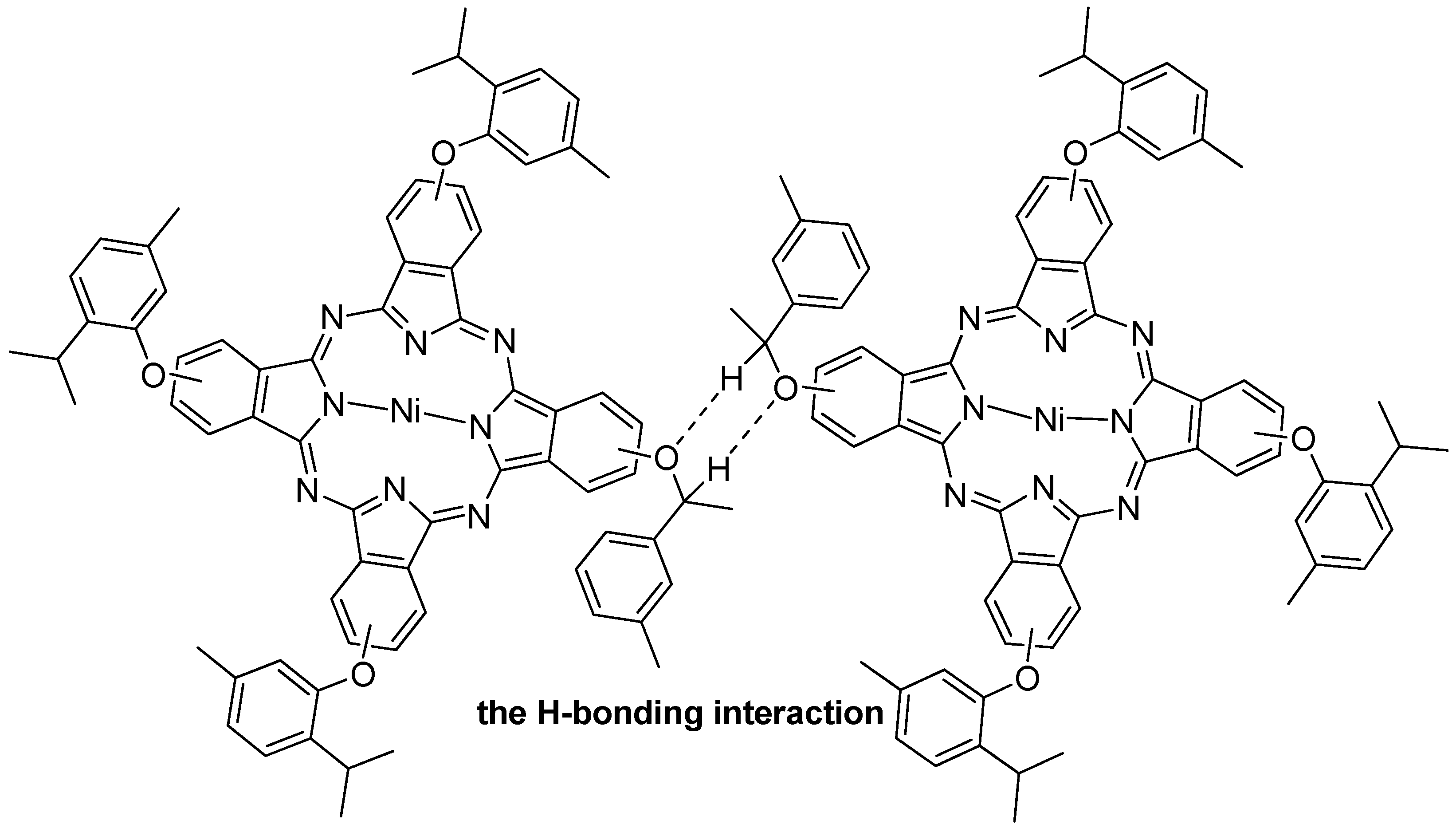
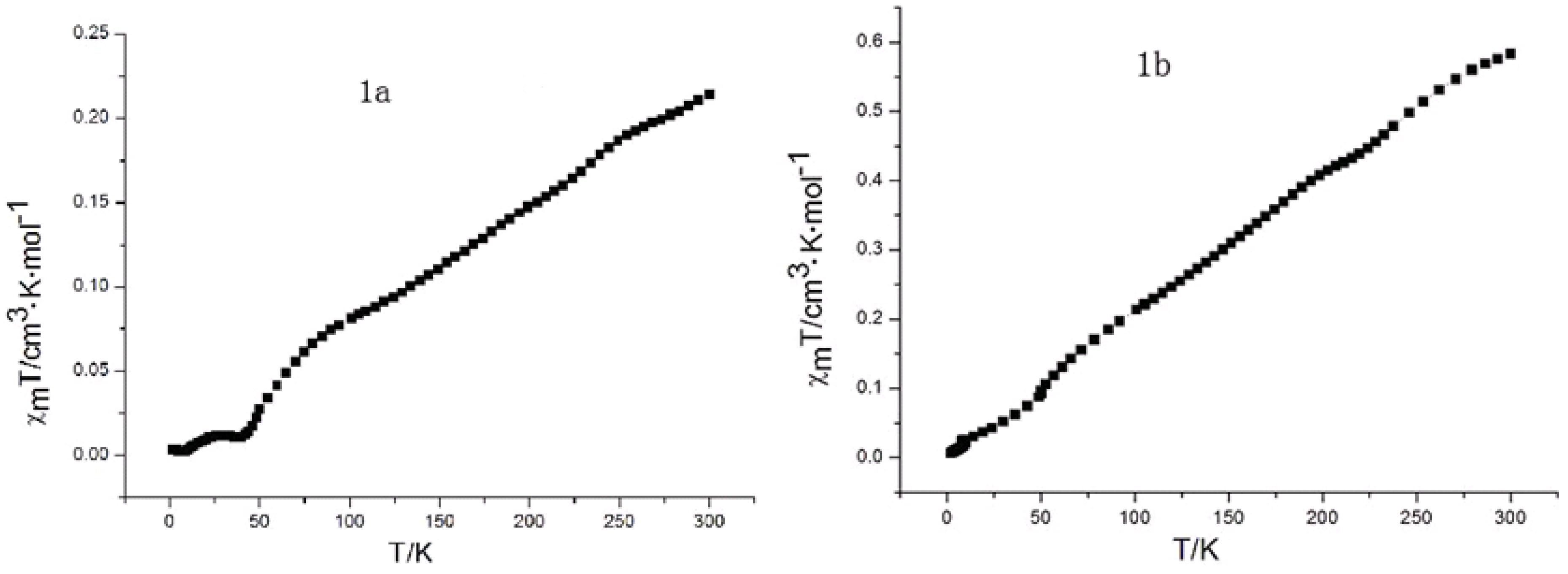
2.7. Magnetic Susceptibility
3. Experimental
3.1. Experimental Materials and Equipment
3.2. Synthesis of Substrates and Pc Derivatives
3.2.1. General Procedure for the Synthesis of Substrates Pn1–Pn3
3.2.2. Synthesis of 1a and 1b
3.3. Synthesis of 1a–3a Nanotubes
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Nabiev, I.; Rakovich, A.; Sukhanova, A.; Lukashev, E.; Zagidullin, V.; Pachenko, V.; Rakovich, Y.P.; Donegan, J.F.; Rubin, A.B.; Govorov, A.O. Fluorescent quantum dots as artificial antennas for enhanced light harvesting and energy transfer to photosynthetic reaction centers. Angew. Chem. Int. Ed. 2010, 49, 7217–7221. [Google Scholar] [CrossRef]
- Kurreck, H.; Huber, M. Model reactions for photosynthesis-photoinduced charge and energy transfer between covalently linked porphyrin and quinone units. Angew. Chem. Int. Ed. 1995, 34, 849–866. [Google Scholar] [CrossRef]
- Hill, J.P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N. Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube. Science 2004, 304, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.D.; Geerts, Y.; Mullen, K.; van de Craats, A.M.; Warman, J.M. Rapid charge transport along self-assembling graphitic nano-wires. Adv. Mater. 1998, 10, 36–38. [Google Scholar] [CrossRef]
- Ishikawa, N.; Kaizu, Y. Synthetic, spectroscopic and theoretical study of novel supramolecular structures composed of lanthanide phthalocyanine double-decker complexes. Coord. Chem. Rev. 2002, 226, 93–101. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ge, X.; Manzano, C.; Kröger, J.; Berndt, R.; Hofer, W.A.; Tang, H.; Cerda, J. Supramolecular patterns controlled by electron interference and direct intermolecular interactions. J. Am. Chem. Soc. 2009, 131, 10400–10402. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Liu, H.B.; Gan, L.B.; Li, Y.L.; Zhu, D. Fabrication of low-dimension nanostructures based on organic conjugated molecules. Adv. Mater. 2008, 20, 2918–2925. [Google Scholar] [CrossRef]
- Liu, H.B.; Xu, J.L.; Li, Y.J.; Li, Y.L. Aggregate nanostructures of organic molecular materials. Acc. Chem. Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N. Phthalocyanine-based magnets. Funct. Phthalocyanine Mol. Mater. 2010, 135, 211–228. [Google Scholar]
- Nyokong, T. Electronic spectral and electrochemical behavior of near infrared absorbing metallophthalocyanines. Funct. Phthalocyanine Mol. Mater. 2010, 135, 45–88. [Google Scholar]
- Zangmeister, R.A.P.; Molenyak, S.P.E.; Drager, A.S.; O’Brien, D.F.; Armstrong, N.R. Transfer of rodlike aggregate phthalocyanines to hydrophobized gold and silicon surfaces: Effect of phenyl-terminated surface modifiers on thin film transfer efficiency and molecular orientation. Langmuir 2001, 17, 7071–7078. [Google Scholar] [CrossRef]
- Ge, X.; Manzano, C.; Berndt, R.; Anger, L.T.; Herges, R. Controlled formation of an axially bonded co-phthalocyanine dimer. J. Am. Chem. Soc. 2009, 131, 6096–6098. [Google Scholar] [CrossRef] [PubMed]
- Gonidec, M.; Luis, F.; Esquena, J.; Amabilino, D.B.; Veciana, J. A liquid-crystalline single-molecule magnet with variable magnetic properties. Angew. Chem. Int. Ed. 2010, 49, 1623–1626. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Sawaguchi, T.; Su, W.; Jiang, J.; Kobayashi, N. Superstructure formation and rearrangement in the adlayer of a rare-earth-metal triple-decker sandwich complex at the electrochemical interface. Angew. Chem. Int. Ed. 2007, 46, 1071–1074. [Google Scholar] [CrossRef]
- Fukada, T.; Sugita, I.; Kobayashi, N. Cation-induced molecular orbital modulations and supramolecular formation of a 15-crown-5-substituted tribenzotetraazachlorin-C60 conjugate. Chem. Commun. 2009, 3449–3451. [Google Scholar] [CrossRef]
- D’Souza, F.; Maligaspe, E.; Ohkubo, K.; Zandler, M.E.; Subbaiyan, N.K.; Fukuzumi, S. Photosynthetic reaction center mimicry: Low reorganization energy driven charge stabilization in self-assembled cofacial zinc phthalocyanine dimer-fullerene conjugate. J. Am. Chem. Soc. 2009, 131, 8787–8797. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura, A.; Martínez-Díaz, M.V.; Thordarson, P.; Rowan, A.E.; Nolte, R.J.M.; Torres, T. Donor-acceptor phthalocyanine nanoaggregates. J. Am. Chem. Soc. 2003, 125, 12300–12308. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Jayawickramarajah, J.; Gouloumis, A.; Pantos, G.D.; Torres, T.; Guldi, D.M. Guanosine and fullerene derived de-aggregation of a new phthalocyanine-linked cytidine derivative. Tetrahedron 2006, 62, 2123–2131. [Google Scholar] [CrossRef]
- Torres, T.; Gouloumis, A.; Sanchez-Garcia, D.; Jayawickramarajah, J.; Seitz, W.; Guldi, D.M.; Sessler, J.L. Photophysical characterization of a cytidine-guanosine tethered phthalocyanine-fullerene dyad. Chem. Commun. 2007, 292–294. [Google Scholar] [CrossRef]
- Liu, L.Y.; Lo, P.C.; Ng, D.K.P. Phthalocyanine-containing supramolecular arrays. Funct. Phthalocyanine Mol. Mater. 2010, 135, 169–210. [Google Scholar]
- Fukuda, T.; Hata, K.; Ishikawa, N. Observation of exceptionally low-lying π–π* excited states in oxidized forms of quadruple-decker phthalocyanine complexes. J. Am. Chem. Soc. 2012, 134, 14698–14701. [Google Scholar] [CrossRef] [PubMed]
- Engelkamp, H.; Middelbeek, S.; Nolte, R.J.M. Self-assembly of disk-shaped molecules to coiled-coil aggregates with tunable helicity. Science 1999, 284, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mauthoor, S.; Din, S.; Gardener, J.A.; Chang, R.; Warner, M.; Aeppli, G.; McComb, D.W.; Ryan, M.P.; Wu, W.; et al. Ultralong copper phthalocyanine nanowires with new crystal structure and broad optical absorption. ACS Nano 2010, 4, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Guldi, D.M.; Gouloumis, A.; Vazquez, P.; Torres, T.; Georgakilas, V.; Prato, M. Nanoscale organization of a phthalocyanine-fullerene system: Remarkable stabilization of charges in photoactive 1-D nanotubules. J. Am. Chem. Soc. 2005, 127, 5811–5813. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Cho, C.-P.; Perng, T.-P. Structural transformation and crystallization of amorphous copper phthalocyanine nanostructures. Thin Solid Films 2010, 518, 6720–6278. [Google Scholar] [CrossRef]
- Luo, Y.; Gao, J.S.; Cheng, C.W.; Sun, Y.F.; Du, X.G.; Xu, G.Y.; Wang, Z.L. Fabrication micro-tube of substituted Zn-phthalocyanine in large scale by simple solvent evaporation method and its surface photovoltaic properties. Org. Electron. 2008, 9, 466–472. [Google Scholar] [CrossRef]
- Gao, J.S.; Zhao, Q.H.; Shi, T.Z.; Huang, G.Q.; Zhang, H.Q. Study on synthesis, characterization and self-assemble nanotubes of a new asymmetrical nickel phthalocyanine. J. Optoelectron. Adv. Mater. 2010, 12, 1562–1565. [Google Scholar]
- Hou, X.K.; Du, X.G.; Ma, C.Y.; Li, Y. Surface photovoltaic properties of an expanded aza H2Pc containing four 1,10-phenanthroline subunits. Synth. Met. 2005, 150, 305–308. [Google Scholar] [CrossRef]
- De la Torre, G.; Bottari, G.; Hahn, U.; Torres, T. Functional phthalocyanines: Synthesis, nanostructuration, and electro-optical applications. Funct. Phthalocyanine Mol. Mater. 2010, 135, 1–44. [Google Scholar]
- Kobayashi, N.; Togashi, M.; Osa, T.; Ishii, K.; Yamauchi, S.; Hino, H. Low symmetrical phthalocyanine analogues substituted with three crown ether voids and their cation-induced supermolecules. J. Am. Chem. Soc. 1996, 118, 1073–1085. [Google Scholar] [CrossRef]
- Li, X.Y.; Sinks, L.E.; Rybtchinski, B.; Wasielewski, M.R. Ultrafast aggregate-to-aggregate energy transfer within self-assembled light-harvesting columns of zinc phthalocyanine tetrakis(perylenediimide). J. Am. Chem. Soc. 2004, 126, 10810–10811. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, H.Z.; Zhu, L.; Zhang, X.B.; Wang, M. Optical absorption and structural studies of erbium biphthalocyanine sublimed films. Mater. Lett. 2003, 57, 4309–4314. [Google Scholar] [CrossRef]
- Tong, W.Y.; Djurisic, A.B.; Xie, M.H.; Ng, A.C.M.; Cheung, K.Y.; Chan, W.K.; Leung, Y.H.; Lin, H.W.; Gwo, S. Metal phthalocyanine nanoribbons and nanowires. J. Phys. Chem B 2006, 110, 17406–17413. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.S.; Watts, J.D.; Huang, M.J. DFT study of unligated and ligated manganeseii porphyrins and phthalocyanines. Inorg. Chem. 2005, 44, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kerridge, A.; Harker, A.H.; Fisher, A.J. Structure-dependent exchange in the organic magnets Cu(II)Pc and Mn(II)Pc. J. Phys. Rev. B 2008, 77, 184403–184415. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.W.; Gao, J.S.; Xu, G.Y.; Zhang, H.Q. Fabricating photoswitches and field-effect transistors from self-assembled tetra(2-isopropyl-5-methyphenoxy) copper phthalocyanines nanowires. J. Nanosci. Nanotechnol. 2009, 9, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, L.B.; Stefan, C.B.M.; Colin, R.; Jessica, M.H.; Xiong, Y.J. Perylenediimide nanowires and their use in fabricating field-effect transistors and complementary inverters. Nano Lett. 2007, 7, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Contact the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, G.; Li, J.; Cong, F.; Li, C.; Chu, X.; Meng, Y.; Du, G.; Du, X. A Series of Asymmetrical Phthalocyanines: Synthesis and Near Infrared Properties. Molecules 2013, 18, 4628-4639. https://doi.org/10.3390/molecules18044628
Huang G, Li J, Cong F, Li C, Chu X, Meng Y, Du G, Du X. A Series of Asymmetrical Phthalocyanines: Synthesis and Near Infrared Properties. Molecules. 2013; 18(4):4628-4639. https://doi.org/10.3390/molecules18044628
Chicago/Turabian StyleHuang, Guoqing, Jianxi Li, Fangdi Cong, Chao Li, Xixi Chu, Yanyan Meng, Guotong Du, and Xiguang Du. 2013. "A Series of Asymmetrical Phthalocyanines: Synthesis and Near Infrared Properties" Molecules 18, no. 4: 4628-4639. https://doi.org/10.3390/molecules18044628