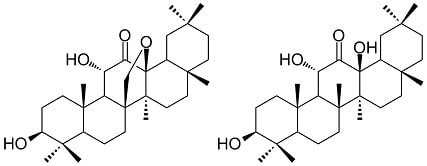
Two New Oleanane-Type Triterpenes Isolated from Japanese Post-Fermented Tea Produced by Anaerobic Microbial Fermentation
Abstract
:1. Introduction
2. Results and Discussion
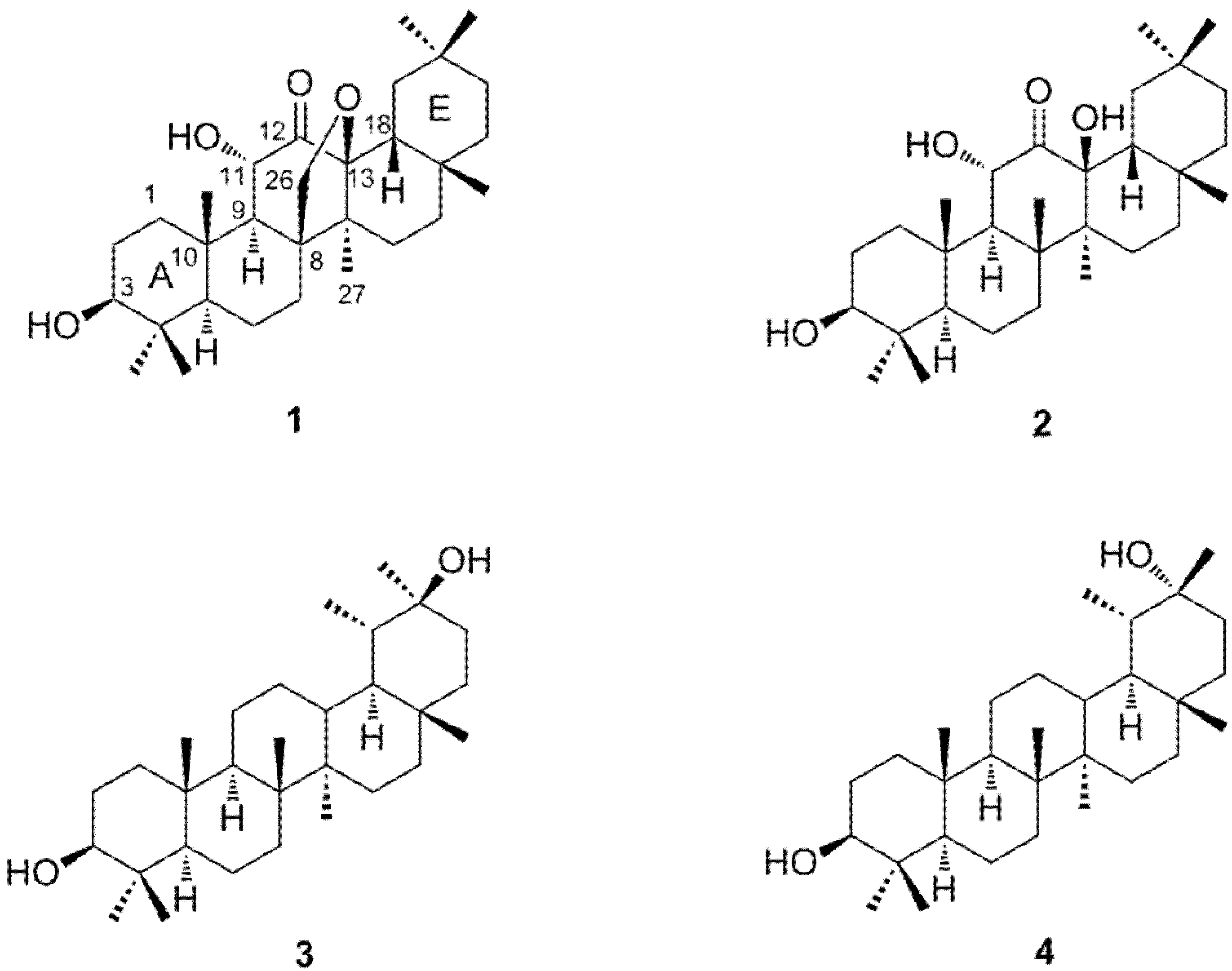
| position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 39.3 | 1.20 dd (15.2, 5.6) | 40.0 | 1.01 m |
| 2.24 m | 2.55 m | |||
| 2 | 27.2 | 1.55 m | 27.5 | 1.54 m |
| 1.62 m | 1.59 m | |||
| 3 | 78.4 | 3.21 dd (11.6, 4.8) | 78.5 | 3.18 dd (10.3, 5.9) |
| 4 | 38.9 | 39.4 | ||
| 5 | 54.7 | 0.75 dd (11.9, 1.9) | 55.3 | 0.70 dd (11.1, 3.3) |
| 6 | 19.5 | 1.09 m | 17.7 | 1.51 m |
| 1.68 m | 1.58 m | |||
| 7 | 29.1 | 1.27 ddd (13.4, 12.8, 4.4) | 34.0 | 1.30 m |
| 1.77 ddd (13.4, 3.0, 2.5) | 1.43 m | |||
| 8 | 50.9 | 43.3 | ||
| 9 | 54.8 | 1.41 dd (10.4, 1.3) | 56.9 | 1.48 d (12.1) |
| 10 | 38.9 | 39.5 | ||
| 11 | 71.8 | 4.30 d (10.4) | 72.4 | 4.89 d (12.1) |
| 12 | 208.4 | 212.7 | ||
| 13 | 89.2 | 82.3 | ||
| 14 | 46.9 | 45.2 | ||
| 15 | 27.8 | 1.12 m | 22.6 | 1.06 m |
| 1.70 m | 2.09 ddd (13.5, 13.2, 4.1) | |||
| 16 | 25.8 | 0.85 m | 30.5 | 1.17 m |
| 1.98 ddd (13.9, 13.7, 4.1) | 1.82 ddd (13.6, 13.4, 4.1) | |||
| 17 | 32.7 | 33.6 | ||
| 18 | 39.7 | 2.02 dd (13.5, 2.5) | 49.0 | 1.50 dd (13.3, 1.8) |
| 19 | 35.6 | 1.09 t (13.5) | 38.5 | 1.25 t (13.3) |
| 2.24 dd (13.5, 2.5) | 1.97 dd (13.3, 1.8) | |||
| 20 | 30.9 | 31.4 | ||
| 21 | 34.5 | 1.12 m | 34.0 | 1.16 m (2H) |
| 1.31 ddd (13.9, 13.4, 4.1) | ||||
| 22 | 38.6 | 1.13 m | 39.0 | 1.21 m |
| 1.59 m | 1.37 m | |||
| 23 | 28.4 | 0.99 s (3H) | 28.2 | 0.99 s (3H) |
| 24 | 15.7 | 0.77 s (3H) | 15.3 | 0.81 s (3H) |
| 25 | 15.8 | 1.09 s (3H) | 16.0 | 1.11 s (3H) |
| 26 | 71.9 | 3.75 dd (9.2, 1.3) | 20.5 | 1.36 s (3H) |
| 4.27 d (9.2) | ||||
| 27 | 16.5 | 0.85 s (3H) | 18.5 | 0.91 s (3H) |
| 28 | 29.4 | 1.00 s (3H) | 31.3 | 1.22 s (3H) |
| 29 | 33.4 | 0.87 s (3H) | 31.9 | 0.89 s (3H) |
| 30 | 23.4 | 0.89 s (3H) | 25.0 | 0.96 s (3H) |
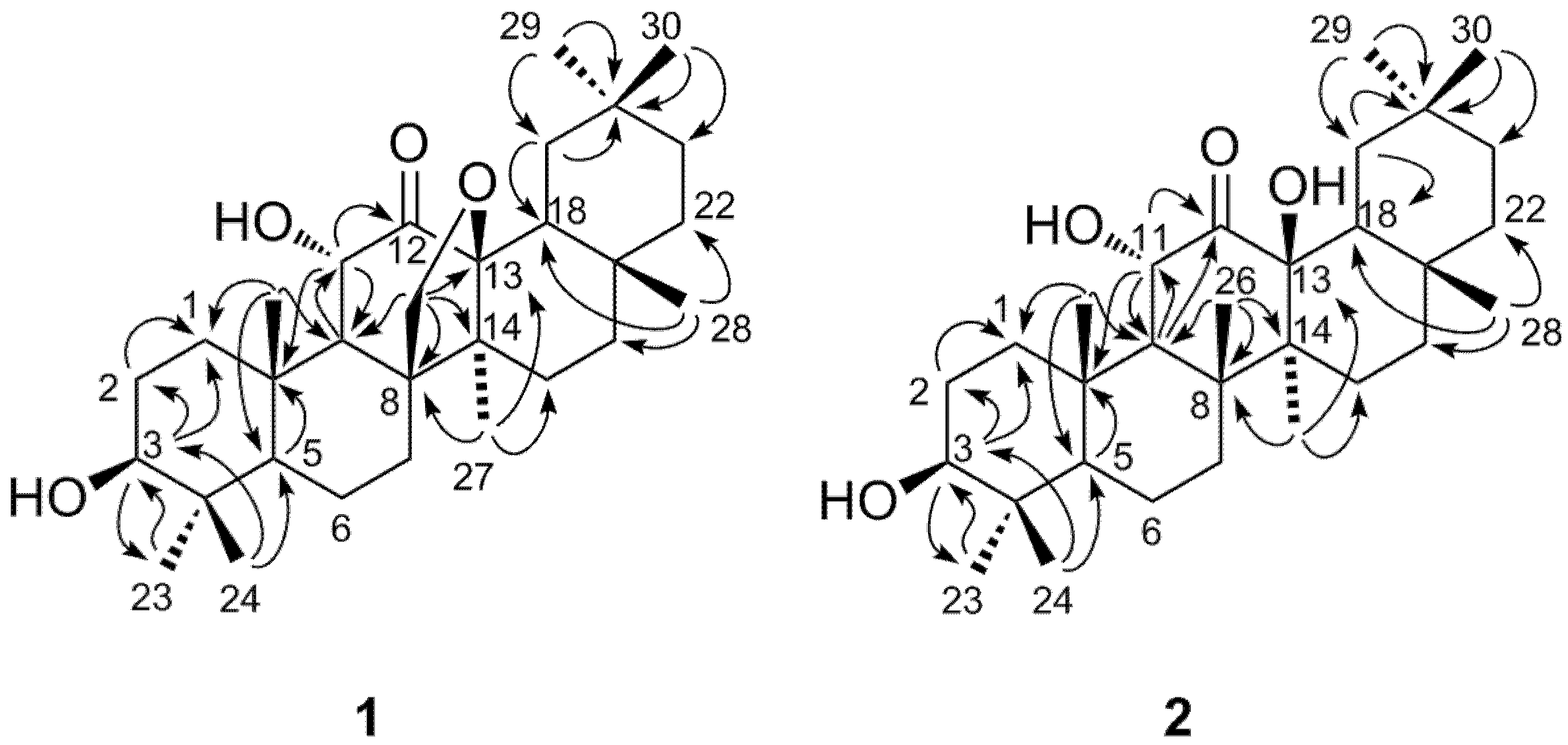
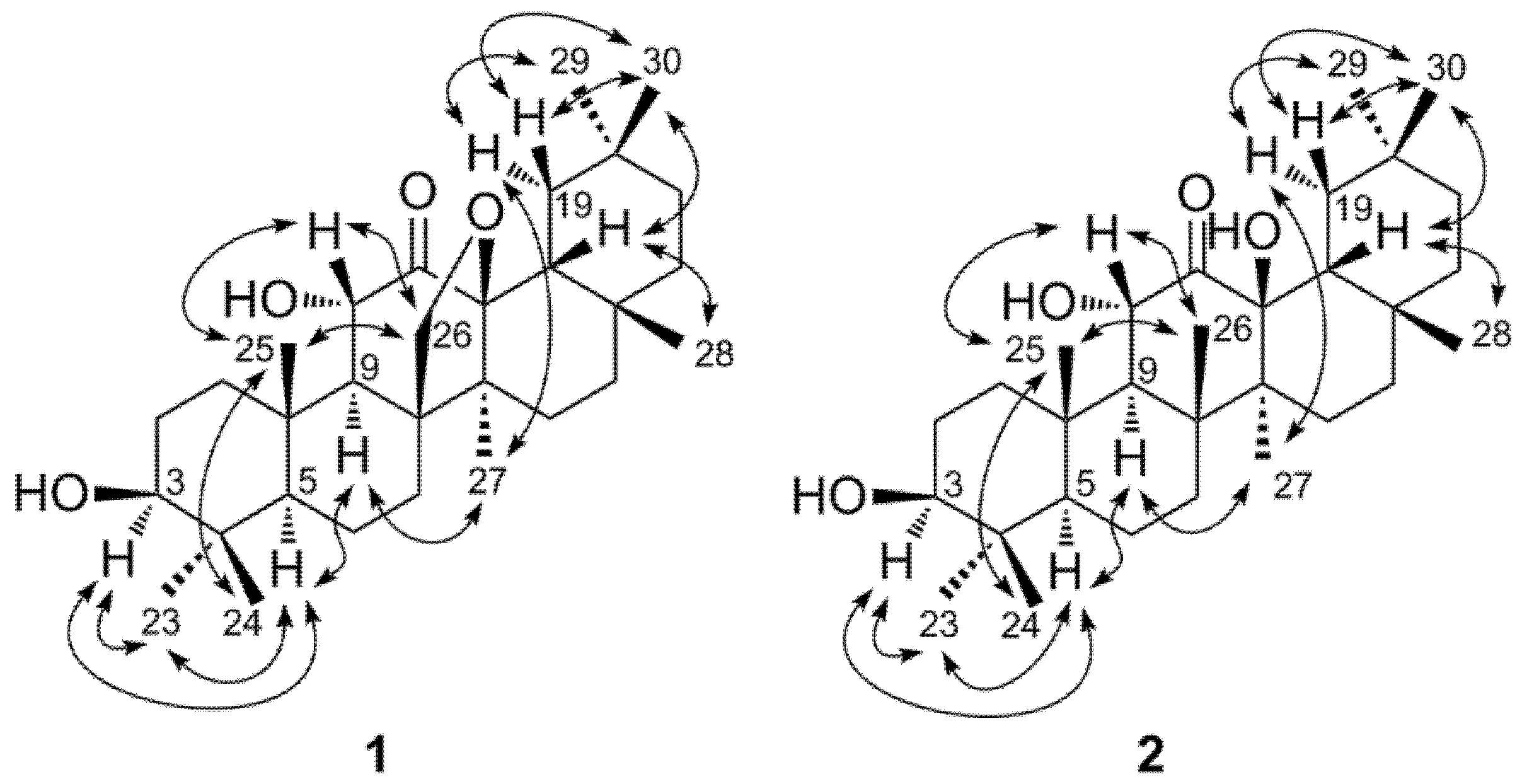
3. Experimental
3.1. Materials
3.2. Analytical Procedures
3.3. Extraction and Separation
3.4. Physicochemical Data of Compounds 1 and 2
4. Conclusions
Acknowledgments
References
- Hara, Y. Green Tea: Health Benefits and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Okada, S.; Takahashi, N.; Ohara, N.; Uchimura, T.; Kozaki, M. Microorganisms involving in the fermentation of Japanese fermented tea leaves. II. Microorganisms in fermentation of Goishi-cha, Japanese fermented tea leaves. J. Jpn. Soc. Food Sci. 1996, 43, 1019–1027. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Shii, T.; Matsuo, Y.; Tanaka, T.; Jiang, Z.-H.; Kouno, I. A new catechin oxidation product and polymeric polyphenols of post-fermented tea. Food Chem. 2011, 129, 830–836. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, Y.J.; Xu, M.; Yang, C.R. Puerins A and B, two new 8-C substituted flavan-3-ols from pu-erh tea. J. Agric. Food Chem. 2005, 53, 8614–8617. [Google Scholar]
- Kanegae, A.; Sakamoto, A.; Nakayama, H.; Nakazono, Y.; Yakashiro, I.; Matsuo, Y.; Tanaka, T.; Ishimaru, K. New phenolic compounds from Camellia sinensis L. fermented leaves. J. Nat. Med. 2012. [Google Scholar] [CrossRef]
- Wulandari, R.A.; Amano, M.; Yanagita, T.; Tanaka, T.; Kouno, I.; Kawamura, D.; Ishimaru, K. New phenolic compounds from Camellia sinensis L. leaves fermented with Aspergillus sp. J. Nat. Med. 2011, 65, 594–597. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Metabolism of (−)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar]
- Tanaka, T.; Nagai, S.; Shii, T.; Matsuo, Y.; Kouno, I. Isolation of 1,3-diphenylpropan-2-ols, identical to tea catechin metabolites produced by intestinal bacteria, and pyrogallol from Japanese post-fermented tea. Jpn. J. Food Chem. Safety 2011, 18, 6–11. [Google Scholar]
- Tanaka, T.; Umeki, H.; Nagai, S.; Shii, T.; Matsuo, Y.; Kouno, I. Transformation of tea catechins and flavonoid glycosides by treatment with Japanese post-fermented tea acetone powder. Food Chem. 2012, 134, 276–281. [Google Scholar] [CrossRef]
- Susunaga, G.S.; Siani, A.C.; Pizzolatti, M.G.; Yunes, R.A.; Monache, F.D. Triterpenes from the resin of Protium heptaphyllum. Fitoterapia 2001, 72, 709–711. [Google Scholar]
- Anjaneyulu, V.; Ravi, K.; Prasad, K.H.; Connolly, J.D. Triterpenoids from Mangifera indica. Phytochemistry 1989, 28, 1471–1477. [Google Scholar]
- Li, X.-H.; Qi, H.-Y.; Shi, Y.-P. Dammarane- and taraxastane-type triterpenoids from Saussurea oligantha Franch. J. Asian Nat. Prod. Res. 2008, 10, 397–402. [Google Scholar]
- Morikawa, T.; Nakamura, S.; Kato, Y.; Muraoka, O.; Matsuda, H.; Yoshikawa, M. Bioactive saponins and glycosides. XXVIII. New triterpene saponins, foliatheasaponins I, II, III, IV, and V, from Tencha (the leaves of Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 293–298. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Li, N.; Nagatomo, A.; Li, X.; Matsuda, H. Bioactive saponins and glycosides. XXIII: triterpene saponins with gastroprotective effect from the seeds of Camellia sinensis: Theasaponins E-3, E-4, E-5, E-6, and E-7. Chem. Pharm. Bull. 2005, 53, 1559–1564. [Google Scholar]
- Herath, H.M.T.B.; Athukoralage, P.S.; Jamie, J.F. Two oleanane triterpenoids from Gordonia ceylanica and their conversions to taraxarane triterpenoids. Phytochemistry 2000, 54, 823–827. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Suzuki, T.; Enjo, F.; Koike, K.; Nikaido, T.; Nishino, H. 3-Epicabraleahydroxylactone and other triterpenoids from camellia oil and their inhibitory effects on epstein-barr virus activation. Chem. Pharm. Bull. 2004, 52, 153–156. [Google Scholar]
- Ling, T.-J.; Wan, X.-C.; Ling, W.-W.; Zhang, Z.-Z.; Xia, T.; Li, D.-X.; Hou, R.-Y. New triterpenoids and other constituents from a special microbial-fermented tea Fuzhuan Brick Tea. J. Agric. Food Chem. 2010, 58, 4945–4950. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Y.-L.; Nagai, S.; Tanaka, T.; Matsuo, Y.; Saito, Y.; Kouno, I. Two New Oleanane-Type Triterpenes Isolated from Japanese Post-Fermented Tea Produced by Anaerobic Microbial Fermentation. Molecules 2013, 18, 4868-4875. https://doi.org/10.3390/molecules18054868
Huang Y-L, Nagai S, Tanaka T, Matsuo Y, Saito Y, Kouno I. Two New Oleanane-Type Triterpenes Isolated from Japanese Post-Fermented Tea Produced by Anaerobic Microbial Fermentation. Molecules. 2013; 18(5):4868-4875. https://doi.org/10.3390/molecules18054868
Chicago/Turabian StyleHuang, Yong-Lin, Sachi Nagai, Takashi Tanaka, Yosuke Matsuo, Yoshinori Saito, and Isao Kouno. 2013. "Two New Oleanane-Type Triterpenes Isolated from Japanese Post-Fermented Tea Produced by Anaerobic Microbial Fermentation" Molecules 18, no. 5: 4868-4875. https://doi.org/10.3390/molecules18054868