Understanding Acid Lability of Cysteine Protecting Groups
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Data
| Abbr. | Protecting group | TFA (%) | [Peptide] (mM) | T (°C) | Reaction time | Deprotected Cys (%) | |
|---|---|---|---|---|---|---|---|
| 1 | 4,4′-diMeODpm |  | 10 | 1 | 25 | 5 min | 100 |
| 10 | 92 | ||||||
| 2 | 4,4′-diMeDpm |  | 20 | 1 | 25 | 5 min | 92 |
| 10 | |||||||
| 70 | |||||||
| 1 | 30 min | 100 | |||||
| 10 | 100 | ||||||
| 3 | Dpm |  | 60 | 1 | 25 | 1 h | 100 |
| 10 | 92 | ||||||
| 4 | 9-F |  | 95 | 1 | 25 | 1 h | 0 |
| 10 | 0 | ||||||
| 5 | 2,6-diMeO-4-MeBn |  | 20 | 1 | 25 | 30 min | 100 |
| 10 | 100 | ||||||
| 6 | 2,4-diMeOBn |  | 20 | 1 | 25 | 5 min | 70 |
| 10 | 5 min | 10 | |||||
| 1 | 30 min | 100 | |||||
| 10 | 30 min | 44 | |||||
| 7 | 2,6-diMe-4-MeOBn |  | 20 | 1 | 25 | 30 min | 100 |
| 10 | 86 | ||||||
| 8 | 2,6-diMeOBn |  | 50 | 1 | 25 | 1 h | 100 |
| 10 | 96 | ||||||
| 9 | 4-MeO-2-MeBn |  | 50 | 1 | 25 | 1 h | 100 |
| 10 | 95 | ||||||
| 10 | Mob |  | 95 | 1 | 25 | 2 h | 35 |
| 10 | 26 | ||||||
| 1 | 40 | 100 | |||||
| 10 | 94 | ||||||
| 11 | TMeb |  | 95 | 1 | 25 | 1 h | 21 |
| 10 | 14 | ||||||
| 12 | biPh |  | 95 | 1 | 25 | 1 h | 0 |
| 10 | 0 | ||||||
| 13 | 2-MeOBn |  | 95 | 1 | 25 | 1 h | 0 |
| 10 | 0 |
2.2. Computational Study


 ,
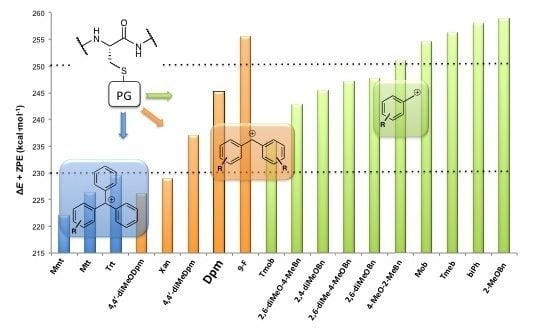
,  , and ER-H were obtained from the calculation of the minimum of energy of the corresponding drawn structures. No imaginary frequencies for minima provide a control that the stationary point localized is correct. Moreover, apart from the ΔE calculation, ΔE + ZPE, ∆H and ∆G were determined. The ∆E + ZPE term is the electronic energy for each process, taking into account the ZPE (Zero Point Energy) correction, and ∆H and ∆G are the corresponding enthalpy and Gibbs energy, respectively. The computational results for Trt, Dpm and Bzl protecting groups are reported in Table 2, Table 3, Table 4, respectively. No solvation effects were considered, and the values are given in kcal·mol–1.
, and ER-H were obtained from the calculation of the minimum of energy of the corresponding drawn structures. No imaginary frequencies for minima provide a control that the stationary point localized is correct. Moreover, apart from the ΔE calculation, ΔE + ZPE, ∆H and ∆G were determined. The ∆E + ZPE term is the electronic energy for each process, taking into account the ZPE (Zero Point Energy) correction, and ∆H and ∆G are the corresponding enthalpy and Gibbs energy, respectively. The computational results for Trt, Dpm and Bzl protecting groups are reported in Table 2, Table 3, Table 4, respectively. No solvation effects were considered, and the values are given in kcal·mol–1.| ΔE | ΔE + ZPE | ΔH | ΔG | |
|---|---|---|---|---|
| Mmt | 228.3 | 222.0 | 223.2 | 217.4 |
| Mtt | 232.9 | 226.4 | 227.6 | 221.9 |
| Trt | 235.8 | 229.4 | 230.6 | 224.7 |
| ΔE | ΔE + ZPE | ΔH | ΔG | |
|---|---|---|---|---|
| 1 | 232.2 | 226.2 | 227.4 | 221.5 |
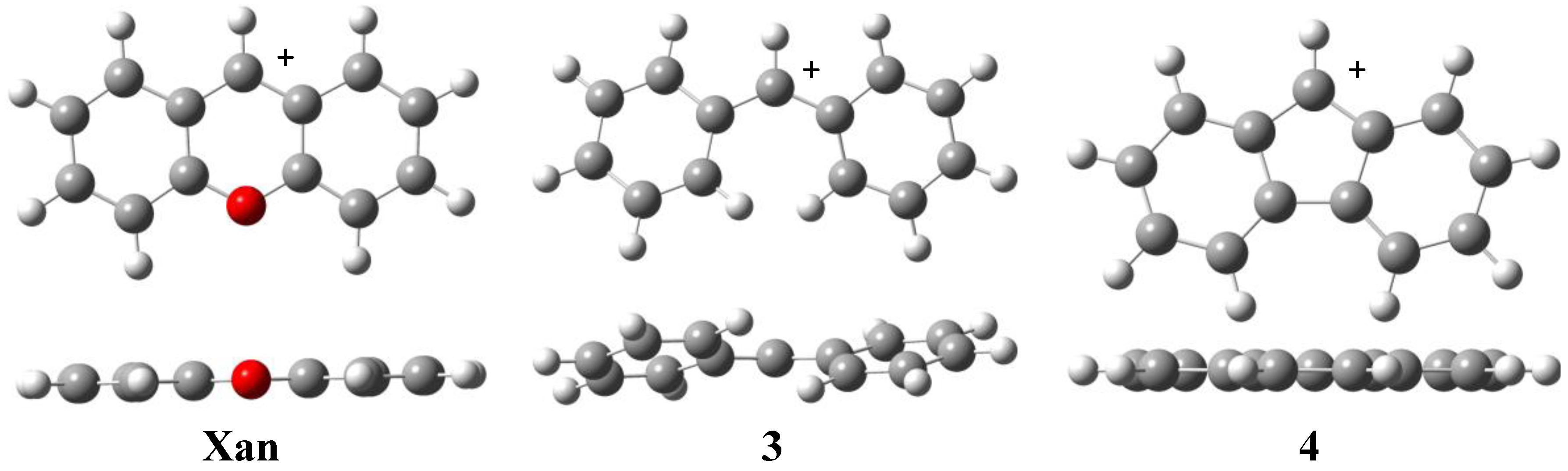
| Xan | 235.2 | 228.9 | 230.1 | 223.6 |
| 2 | 243.6 | 237.1 | 238.3 | 232.4 |
| 3 | 251.7 | 245.2 | 246.5 | 240.6 |
| 4 | 262.5 | 255.5 | 256.9 | 249.2 |
| ΔE | ΔE + ZPE | ΔH | ΔG | |
|---|---|---|---|---|
| Tmob | 241.9 | 235.8 | 236.9 | 230.5 |
| 5 | 249.1 | 242.8 | 243.9 | 237.6 |
| 6 | 251.8 | 245.4 | 246.6 | 239.5 |
| 7 | 253.4 | 247.1 | 248.2 | 241.9 |
| 8 | 254.0 | 247.7 | 248.8 | 242.5 |
| 9 | 257.7 | 251.1 | 252.4 | 245.0 |
| 10 (Mob) | 261.0 | 254.6 | 255.7 | 249.5 |
| 11 | 263.2 | 256.3 | 257.5 | 250.5 |
| 12 | 264.5 | 258.0 | 259.1 | 253.2 |
| 13 | 265.4 | 258.8 | 260.1 | 252.9 |

3. Experimental
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino acid-protecting groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [Green Version]
- Góngora-Benítez, M.; Basso, A.; Bruckdorfer, T.; Royo, M.; Tulla-Puche, J.; Albericio, F. Eco-Friendly Combination of the Immobilized PGA Enzyme and the S-Phacm Protecting Group for the Synthesis of Cys-Containing Peptides. Chem. Eur. J. 2012, 18, 16166–16176. [Google Scholar]
- Postma, T.M.; Giraud, M.; Albericio, F. Trimethoxyphenylthio as a highly labile replacement for tert-butylthiocysteine protection in Fmoc solid phase synthesis. Org. Lett. 2012, 14, 5468–5471. [Google Scholar] [CrossRef]
- Akabori, S.; Sakakibara, S.; Shimonishi, Y.; Nabuhara, Y. A New Method for the Protection of the Sulfhydryl Group during Peptide Synthesis. Bull. Chem. Soc. Jpn. 1964, 37, 433–434. [Google Scholar] [CrossRef]
- Harris, K.M.; Flemer, S., Jr.; Hondal, R.J. Studies on deprotection of cysteine and selenocysteine side-chain protecting groups. J. Pept. Sci. 2007, 13, 81–93. [Google Scholar] [CrossRef]
- Although, Dpm was first reported in the 70’s by Photaki and co-workers in that moment its practical use in peptide synthesis was discarded because it was described the need of boiling TFA to remove it, probably due to the less effective scavenger applied. Photaki, I.; Taylor-Papadimitrious, J.; Sakarellos, C.; Mazarakis, P.; Zervas, L. J. Chem. Soc. 1970, 2683.
- Góngora-Benítez, M.; Mendive-Tapia, L.; Ramos-Tomillero, I.; Breman, A.C.; Tulla-Puche, J.; Albericio, F. Acid-labile Cys-protecting groups for the Fmoc/tBu strategy: Filling the gap. Org. Lett. 2012, 14, 5472–5475. [Google Scholar] [CrossRef]
- James, I.A. Linkers for solid phase organic synthesis. Tetrahedron 1999, 55, 4855–4946. [Google Scholar] [CrossRef]
- Guillier, F.; Orain, D.; Bradley, M. Linkers and cleavage strategies in solid-phase organic synthesis and combinatorial chemistry. Chem. Rev. 2000, 100, 2091–2157. [Google Scholar] [CrossRef]
- Hoffman, R.; Bissell, R.; Farnum, D.G. The balance of steric and conjugative effects in phenyl-substituted cations, radicals, and anions. J. Phys. Chem. 1969, 73, 1789–1800. [Google Scholar] [CrossRef]
- Rodriguez, C.F.; Vucković, D.L.; Hopkinson, A.C. Benzyl, 9-fluorenyl and diphenylmethyl cations: Structures and relative stabilities based on hydride transfer reactions. J. Mol. Struct. (Theochem) 1996, 363, 131–138. [Google Scholar] [CrossRef]
- Fernández, I.; Frenking, G. Correlation between Hammett substituent constants and directly calculated π-conjugation strength. J. Org. Chem. 2006, 71, 2251–2256. [Google Scholar] [CrossRef]
- Data in absence of scavengers are not shown.
- Barlos, K.; Gatos, D.; Hatzi, O.; Koch, N.; Koutsogianni, S. Synthesis of the very acid-sensitive Fmoc-Cys(Mmt)-OH and its application in solid-phase peptide synthesis. Int. J. Pept. Protein Res. 1996, 47, 148–153. [Google Scholar]
- Sax, B.; Dick, F.; Tanner, R.; Gosteli, J. 4-Methyltrityl (Mtt): A new protecting group for the side chain protection of Asn and Gln in solid-phase peptide synthesis. Pept. Res. 1992, 5, 245–246. [Google Scholar]
- Han, Y.; Barany, G. Novel S-Xanthenyl Protecting Groups for Cysteine and Their Applications for the Nα-9-Fluorenylmethyloxycarbonyl (Fmoc) Strategy of Peptide Synthesis. J. Org. Chem. 1997, 62, 3841–3848. [Google Scholar] [CrossRef]
- Munson, M.C.; Garcia-Echeverria, C.; Albericio, F.; Barany, G. S-2,4,6-trimethoxybenzyl (Tmob): A novel cysteine protecting group for the Nα-(9-fluorenylmethoxycarbonyl) (Fmoc) strategy of peptide synthesis. J. Org. Chem. 1992, 57, 3013–3018. [Google Scholar] [CrossRef]
- Hiskey, R.G.; Adams, J.B. Sulfur-containing polypeptides. IV. Synthetic routes to cysteine peptides. J. Org. Chem. 1966, 31, 2178–2183. [Google Scholar] [CrossRef]
- Garcia, O.; Bofill, J.M.; Nicolas, E.; Albericio, F. 2,2,4,6,7-Pentamethyl-2,3-dihydrobenzofuran-5-methyl (Pbfm) as an alternative to the trityl group for the side-chain protection of Cysteine and Asparagine/Glutamine. Eur. J. Org. Chem. 2010, 19, 3631–3640. [Google Scholar]
- Pittelkow, M.; Christensen, J.B.; Sølling, T.I. Substituent effects on the stability of extended benzyliccarbocations: A computational study of conjugation. Org. Biomol. Chem. 2005, 3, 2441–2449. [Google Scholar] [CrossRef]
- Mladenova, G.; Chen, L.; Rodriguez, C.F.; Siu, K.W.M.; Johnston, L.J.; Hopkinson, A.C.; Lee-Ruff, E. Studies of 9-fluorenyl carbocations. Intramolecular hydride migration in substituted 9-fluorenyl carbocation. J. Org. Chem. 2001, 33, 1109–1114. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ramos-Tomillero, I.; Mendive-Tapia, L.; Góngora-Benítez, M.; Nicolás, E.; Tulla-Puche, J.; Albericio, F. Understanding Acid Lability of Cysteine Protecting Groups. Molecules 2013, 18, 5155-5162. https://doi.org/10.3390/molecules18055155
Ramos-Tomillero I, Mendive-Tapia L, Góngora-Benítez M, Nicolás E, Tulla-Puche J, Albericio F. Understanding Acid Lability of Cysteine Protecting Groups. Molecules. 2013; 18(5):5155-5162. https://doi.org/10.3390/molecules18055155
Chicago/Turabian StyleRamos-Tomillero, Iván, Lorena Mendive-Tapia, Miriam Góngora-Benítez, Ernesto Nicolás, Judit Tulla-Puche, and Fernando Albericio. 2013. "Understanding Acid Lability of Cysteine Protecting Groups" Molecules 18, no. 5: 5155-5162. https://doi.org/10.3390/molecules18055155