Retention of Halogenated Solutes on Stationary Phases Containing Heavy Atoms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Functional Groups Contents
| Adsorbents | Contents of phenoxy groups/mmol g−1 |
|---|---|
| Pentabromophenoxy (PBP) | 0.30 |
| 2,4,6-Tribromophenoxy (TBP) | 0.23 |
| 2,4-Dibromophenoxy (DBP) | 0.34 |
| 4-Bromophenoxy (4BP) | 0.43 |
| 2,4-Dichlorophenoxy (DCP) | 0.56 |
| Phenoxy (Phe) | 1.22 |
2.2. SPE Efficiencies of the Synthesized Adsorbents
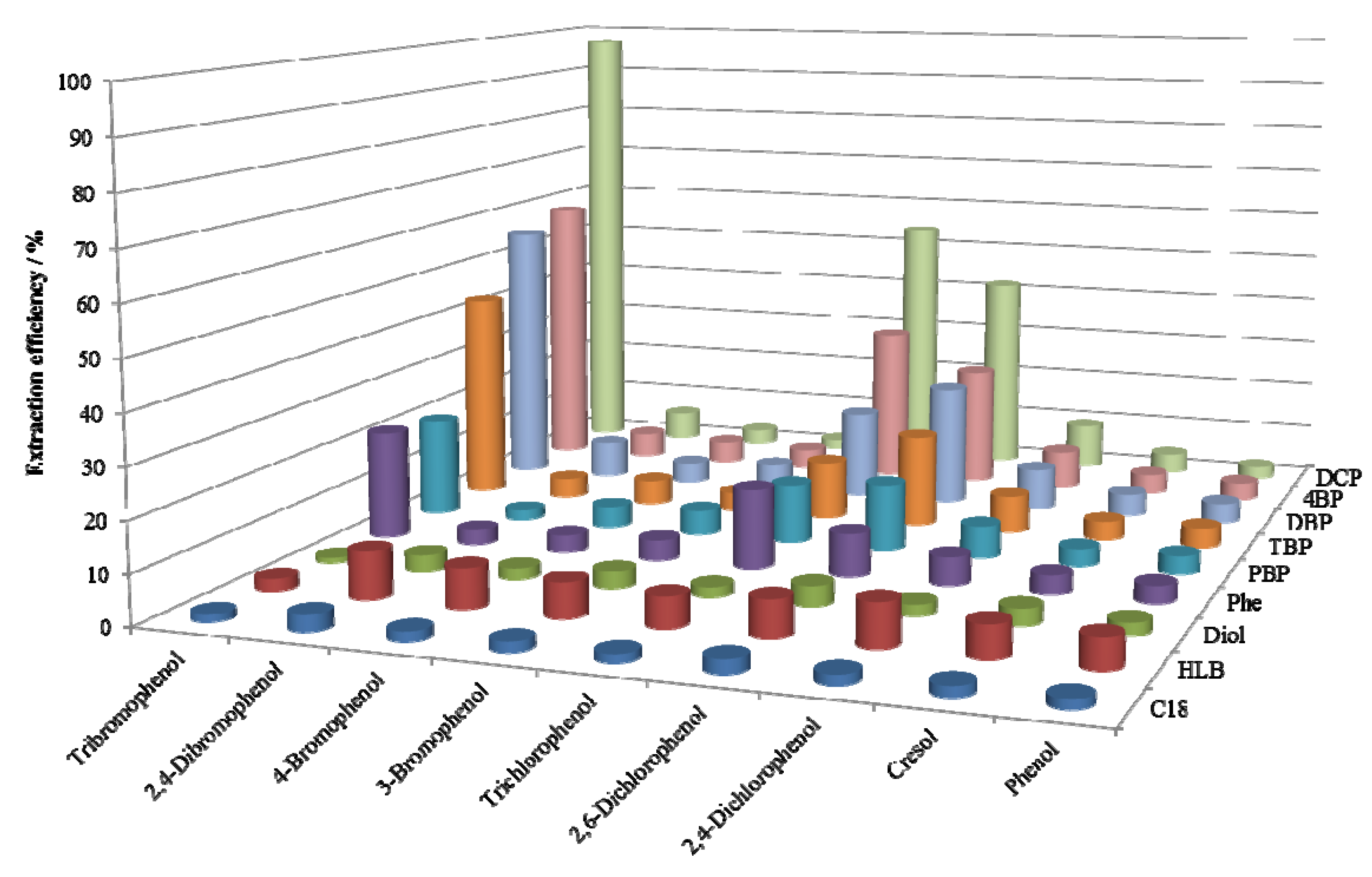
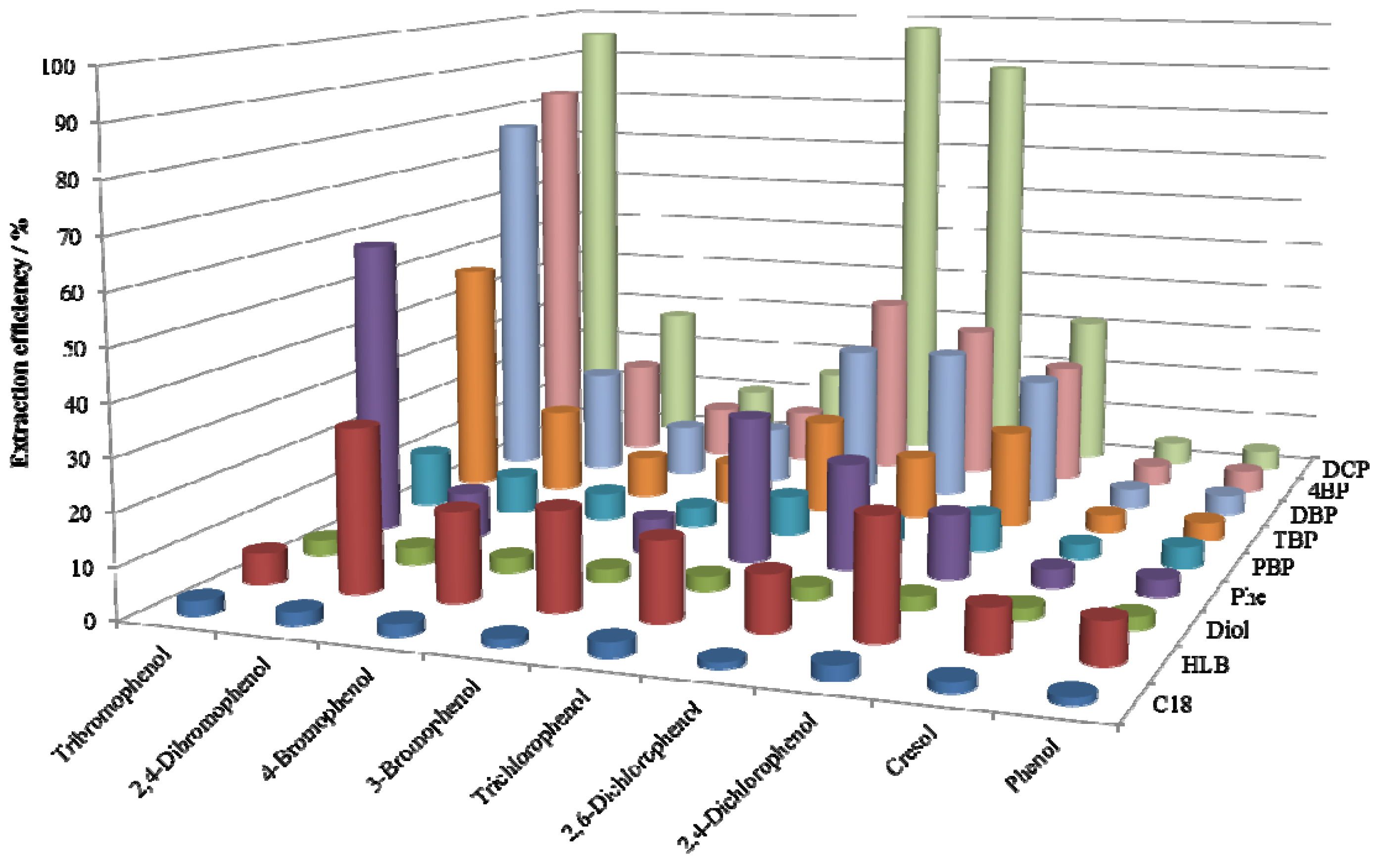
2.3. Effect of Orientation Dipole Moments of Functional Groups on the Retention
| Phenols | Dipole moment/D | References |
|---|---|---|
| 2,4-Dichlorophenol | 3.019 * | [15] |
| 2,4-Dibromophenol | 2.64–2.99 * | [16,17] |
| 4-Bromophenol | 2.15–2.78 | [18,19] |
| 2,4,6-Tribromophenol | 1.44–2.15 | [18,20] |
| Pentabromophenol | 1.73 | [16] |
| Phenol | 1.22–1.86 | [18,21] |
2.4. Effect of Induced Dipole Moments of Solutes on Retention
| Phenols | Dipole moment/D | Refractive index | Specific volume/g cm−3 | Molar weight/g mol−1 | Molar refraction/cm3 mol−1 |
|---|---|---|---|---|---|
| 2,4,6-Tribromophenol | 1.45–2.15 | 1.7 a | 2.55 (20 °C) | 330.8 | 50.1 (51.2 b) |
| 2,4,6-Trichlorophenol | 1.38–2.00 [18,20] | 1.6 a | 1.675 | 197.45 | 40.3 (42.8 b) |
| 2,6-Dichlorophenol | 1.77–2.15 [15,21] | 1.594 b | 1.532 | 163.0 | 36.1 (37.9 b) |
| 2,4-Dichlorophenol | 0.846–3.019 | 1.594 b | 1.383 | 163.0 | 40.0 (37.9 b) |
| 2,4-Dibromophenol | 0.37–2.99 | 1.643 b | 2.07 (20 °C) | 251.9 | 44.0 (43.5 b) |
| 3-Bromophenol | 0.90–3.10 [16] | 1.5957 | 1.63 | 173.0 | 36.2 (35.8 b) |
| 4-Bromophenol | 2.15–2.78 | 1.5875 | 1.65 | 173.0 | 35.3 (35.8 b) |
| 4-Cresol | 1.44–1.83 [18] | 1.5312 | 1.0347 | 108.1 | 33.1 (33.0 b) |
| Phenol | 1.22–1.86 | 1.5425 | 1.07 | 94.1 | 27.7 (28.1 b) |
3. Experimental
3.1. Reagents and Chemicals
3.2. Synthesis and Characterization of the Adsorbents with Heavy Atom Effect
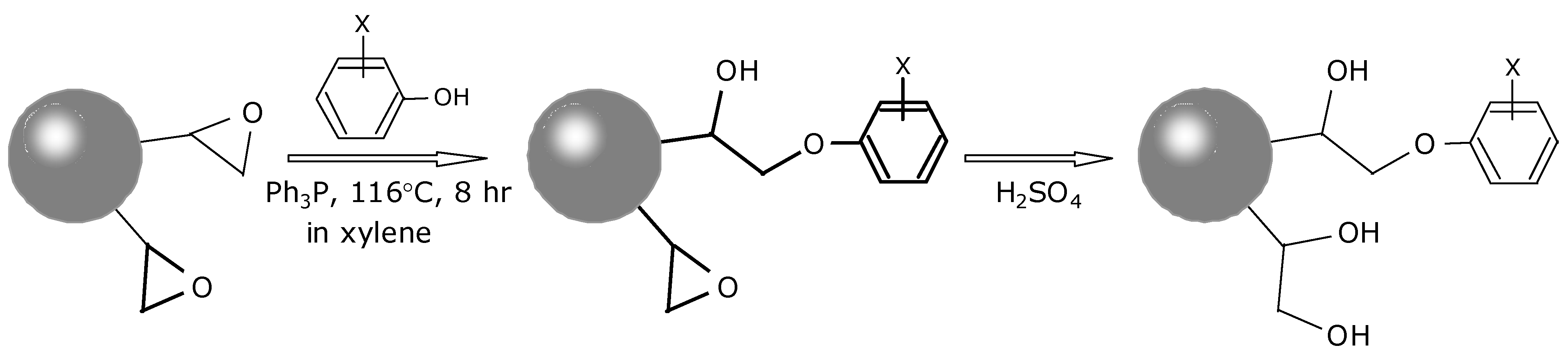
3.3. Evaluation of the SPE Adsorbents
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Majors, R.E. Advanced topics in solid-phase extraction. LC GC Europe 2007, 20, 266–279. [Google Scholar]
- Żwir-Ferenc, A.; Biziuk, M. Solid phase extraction technique—Trends, opportunities and applications. Polish J. Environ. Stud. 2006, 15, 677–690. [Google Scholar]
- Poole, F.C. New trends in solid-phase extraction. Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Rawa-Adkonis, M.; Wolska, L.; Namieśnik, J. Modern techniques of extraction of organic analytes from environmental matrices. Critic. Rev. Anal. Chem. 2003, 33, 199–248. [Google Scholar] [CrossRef]
- Masque, N.; Marce, R.M.; Borrull, F. New polymeric and other types of sorbents for solid-phase extraction of polar organic micropollutants from environmental water. Trends Anal. Chem. 1998, 6, 384–394. [Google Scholar] [CrossRef]
- Xu, Z.X.; Gao, H.J.; Zhang, L.M.; Chen, X.Q.; Qiao, X.G. The biomimetic immunoassay based on molecularly imprinted polymer: A comprehensive review of recent progress and future prospects. J. Food Sci. 2011, 76, R69–R75. [Google Scholar] [CrossRef]
- Haginaka, J. Selectivity of affinity media in solid-phase extraction of analytes. Trends Anal. Chem. 2005, 24, 407–415. [Google Scholar] [CrossRef]
- McGrath, T.F.; Elliott, C.T.; Fodey, T.L. Biosensors for the analysis of microbiological and chemical contaminants in food. Anal. Bioanal. Chem. 2012, 403, 75–92. [Google Scholar] [CrossRef]
- Iliuk, A.B.; Hu, L.; Tao, W.A. Aptamer in bioanalytical applications. Anal. Chem. 2011, 83, 4440–4452. [Google Scholar] [CrossRef]
- Juskowiak, B. Nucleic acid-based fluorescent probes and their analytical potential. Anal. Bioanal. Chem. 2011, 399, 3157–3176. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Shimizu, M.; Yamamoto, A.; Kodama, S.; Kamichatani, W.; Inoue, Y. Development of a novel multi-functional adsorbent equipped with long chain hydrophobic and anion exchange groups and its application to simple and rapid determination of residual acephate in vegetables (in Japanese). Shokuhinn Eisei Gakkaishi 2010, 51, 58–64. [Google Scholar] [CrossRef]
- Kimata, K.; Hirose, T.; Moriuchi, K.; Hosoya, K.; Araki, T.; Tanaka, N. High-capacity stationary phases containing heavy atoms for HPLC separation of fullerenes. Anal. Chem. 1995, 67, 2556–2561. [Google Scholar]
- Kimata, K.; Hosoya, K.; Tanaka, N.; Barnart, E.R.; Alexander, L.R.; Patterson, D.G., Jr. Evaluation of nitrophenyl-bonded silica for reversed-phase liquid chromatography and its application to the separation of polychlorinated dibenzo-p-dioxin isomers (in Japanese). Bunseki Kagaku 1993, 42, 837–843. [Google Scholar]
- Turowski, M.; Yamanaka, N.; Meller, J.; Kimata, K.; Ikegami, T.; Hosoya, K.; Tanaka, N.; Thornton, E. Deuterium isotope effect on hydrophobic interactions: The importance of dispersion interactions in the hydrophobic phase. J. Am. Chem. Soc. 2003, 125, 13836–13849. [Google Scholar]
- Han, J.; Deming, R.L.; Tao, F.-M. Theoretical study of molecular structures and properties of the complete series of chlorophenols. J. Phys. Chem. A 2004, 108, 7736–7743. [Google Scholar]
- Han, J.; Lee, H.; Tao, F.-M. Molecular structures and properties of the complete series of bromophenols: Density functional theory calculations. J. Phys. Chem. A 2005, 109, 5186–5192. [Google Scholar] [CrossRef]
- Anthony, A.A.; Fong, F.K.; Smyth, C.P. Microwave absorption and molecular structure in liquids. LVIII. The dielectric relaxations, infrared spectra, and intramolecular hydrogen bonding of 2,6-dichloro-p-nitroaniline and four substituted phenols. J. Phys. Chem. 1964, 68, 2035–2039. [Google Scholar]
- Erić, B.; Goode, E.V.; Ibbitson, D.A. Studies of dipole moments, and sorption characteristics at the solution-solid interface, of a series of substituted phenols. J. Org. Chem. 1960, 55–61. [Google Scholar]
- Anzilotti, W.F.; Curran, B.C. Electric moments of ortho-substituted phenols and anisoles. I. Halogen derivatives. J. Am. Chem. Soc. 1943, 65, 607–611. [Google Scholar] [CrossRef]
- Kraft, J.; Walker, S.; Magee, M.D. Some dielectric and spectroscopic studies of molecular interaction of some 2,6-dihalo-substituted phenols with tertiary amines. J. Phys. Chem. 1975, 79, 881–885. [Google Scholar] [CrossRef]
- Yogesh, P.; Popat, K.M.; Ganguly, B.; Brahmbhatt, H.; Bhattacharya, A. Studies on the separation performances of chlorophenol compounds from water by thin film composite membranes. Macromol. Res. 2008, 16, 590–595. [Google Scholar] [CrossRef]
- Inoue, Y.; Kamichatani, W.; Saito, M.; Kobayashi, Y.; Yamamoto, A. Extraction and separation properties of hydrophilic compounds using novel water-holding adsorbents bonded with a zwitter-ionic polymer. Chromatographia 2011, 73, 849–855. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the adsorbents are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miwa, T.; Yamamoto, A.; Saito, M.; Inoue, Y. Retention of Halogenated Solutes on Stationary Phases Containing Heavy Atoms. Molecules 2013, 18, 5163-5171. https://doi.org/10.3390/molecules18055163
Miwa T, Yamamoto A, Saito M, Inoue Y. Retention of Halogenated Solutes on Stationary Phases Containing Heavy Atoms. Molecules. 2013; 18(5):5163-5171. https://doi.org/10.3390/molecules18055163
Chicago/Turabian StyleMiwa, Toshio, Atsushi Yamamoto, Mitsuru Saito, and Yoshinori Inoue. 2013. "Retention of Halogenated Solutes on Stationary Phases Containing Heavy Atoms" Molecules 18, no. 5: 5163-5171. https://doi.org/10.3390/molecules18055163




