Antagonist Effects of Veratric Acid against UVB-Induced Cell Damages
Abstract
:1. Introduction
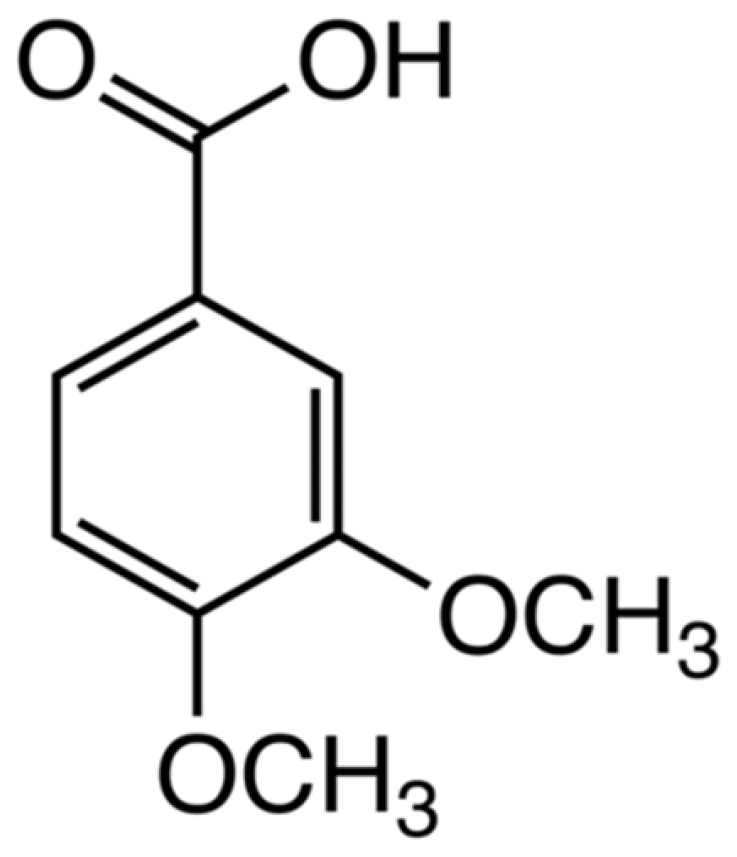
2. Results and Discussion
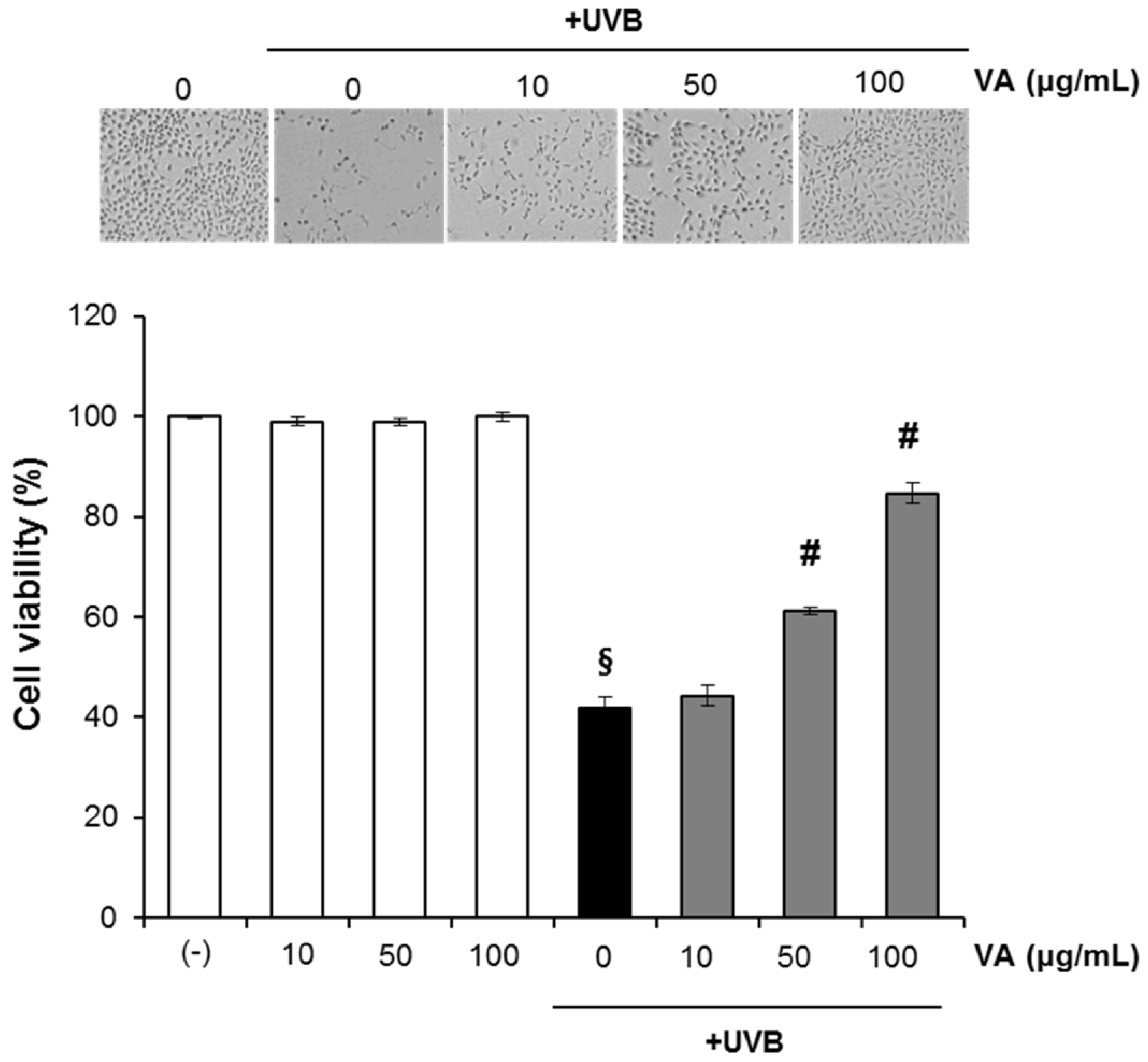
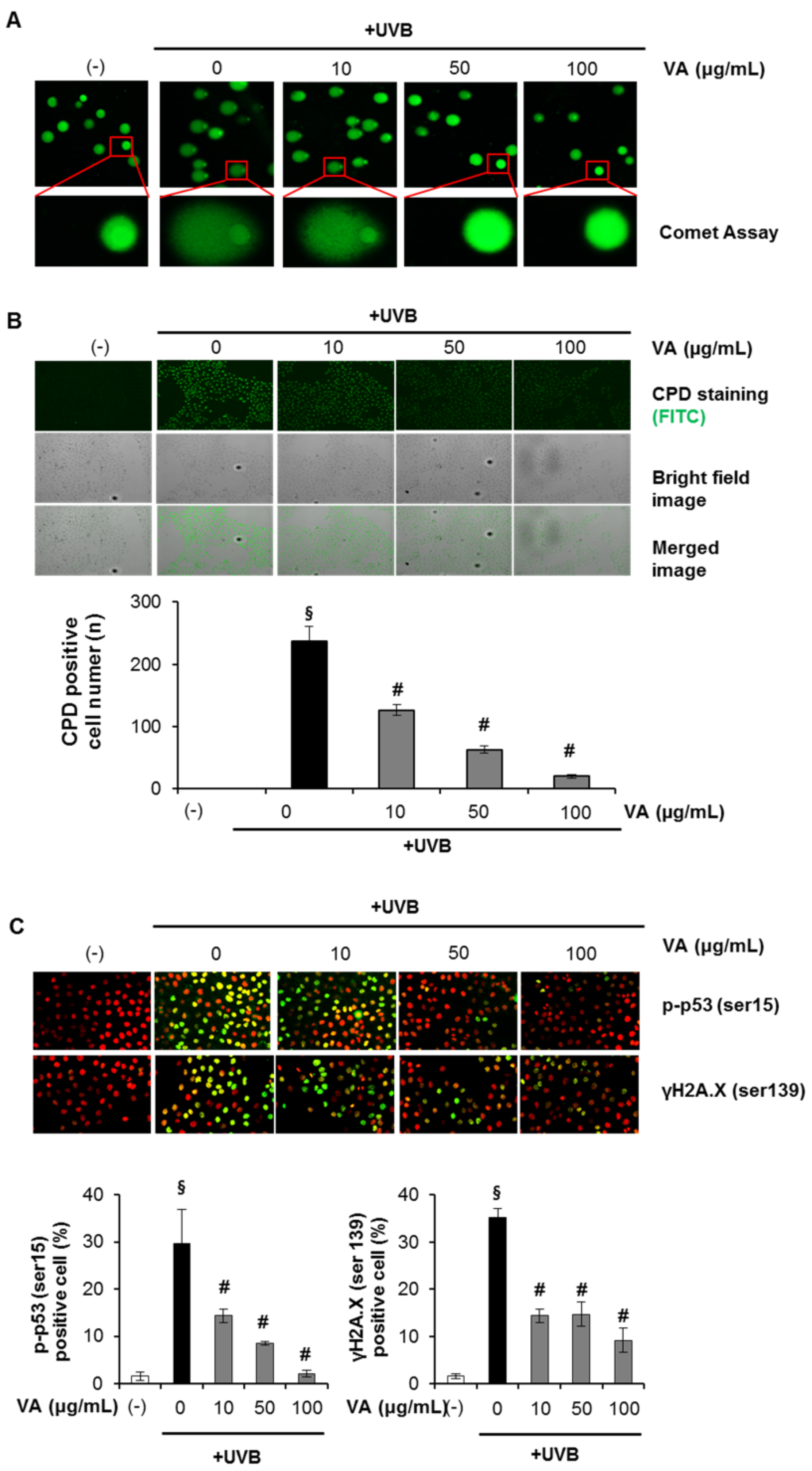
2.1. Veratric Acid Inhibits UV-Induced Damage in HaCaT Cells
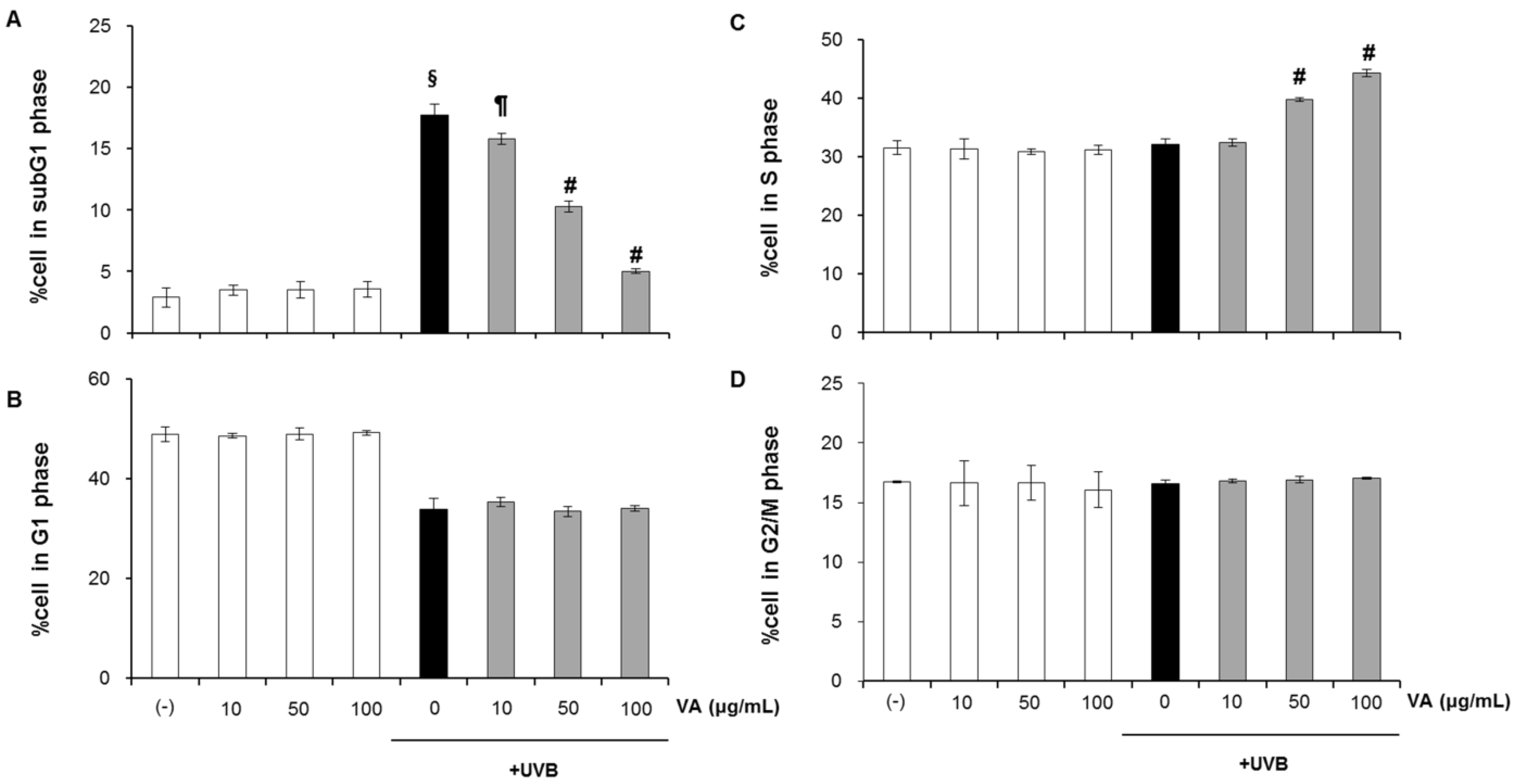
2.2. Veratric Acid Increases S-Phase Population in UVB-Irradiated Cells
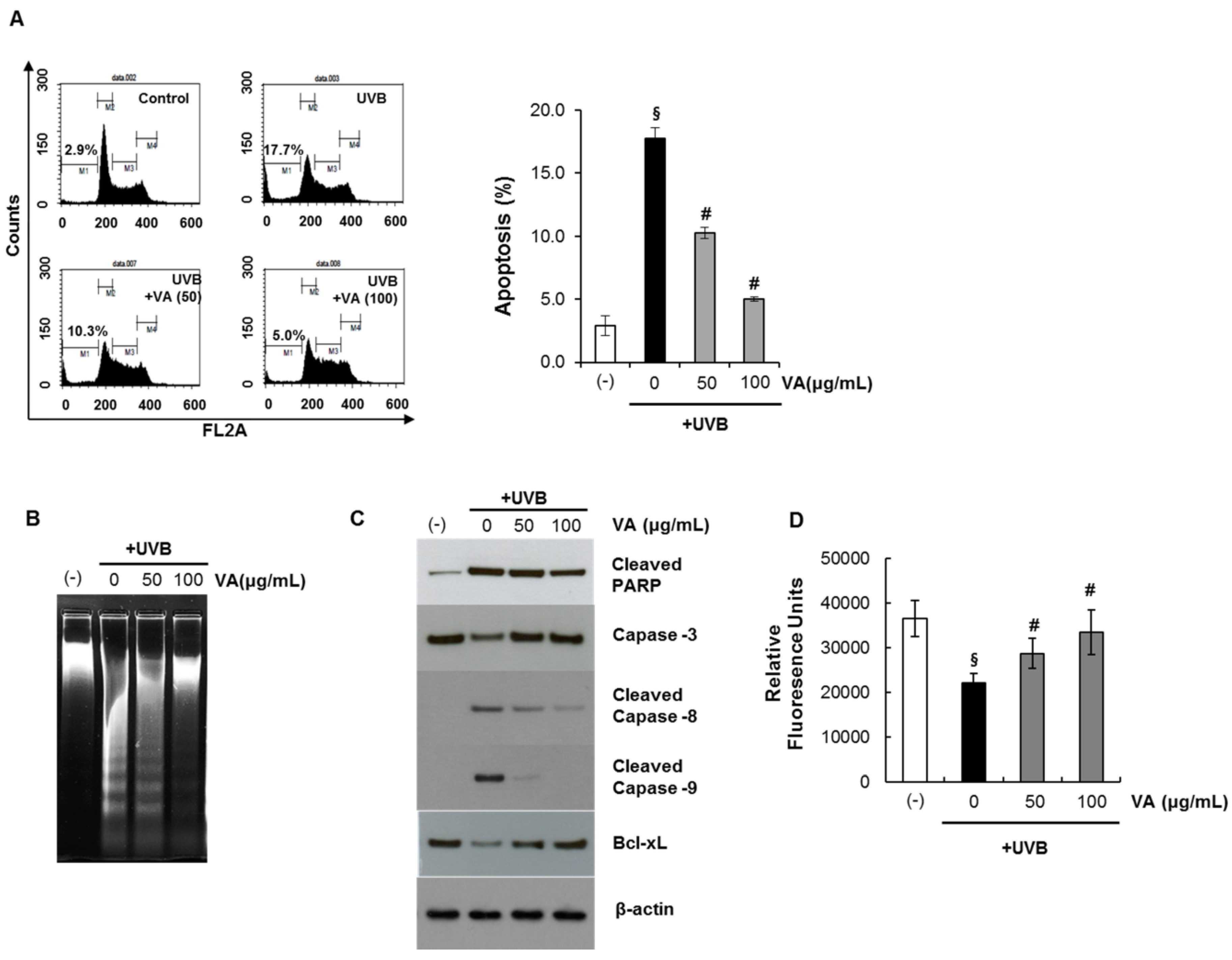
2.3. Veratric Acid Protects HaCaT Cells from UVB-Mediated Apoptosis
2.4. Veratric Acid Prevent UVB-Induced Depletion of GSH in HaCaT Cells
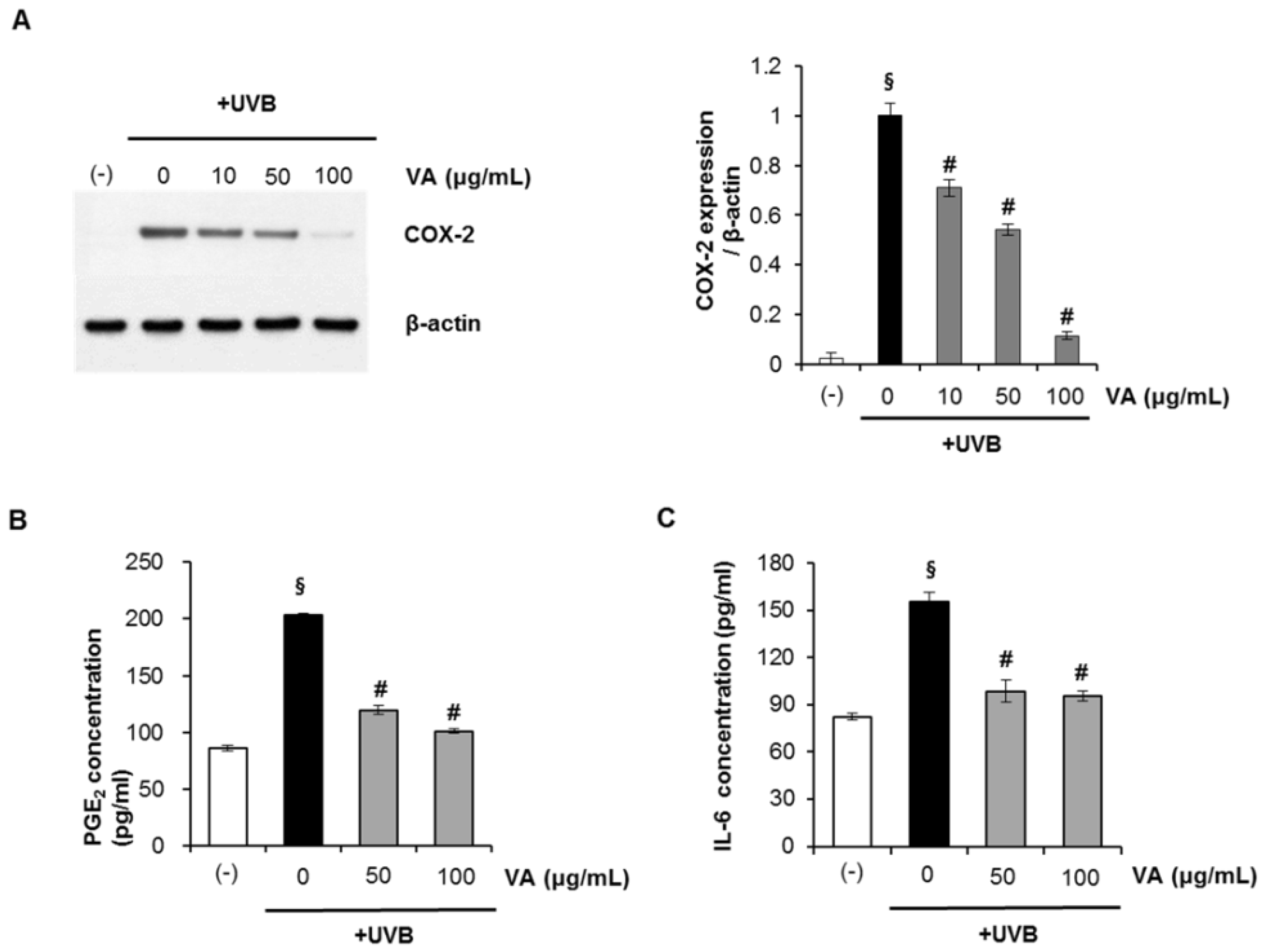
2.5. Veratric Acid Inhibits UVB-induced Inflammation in Vitro and in Vivo
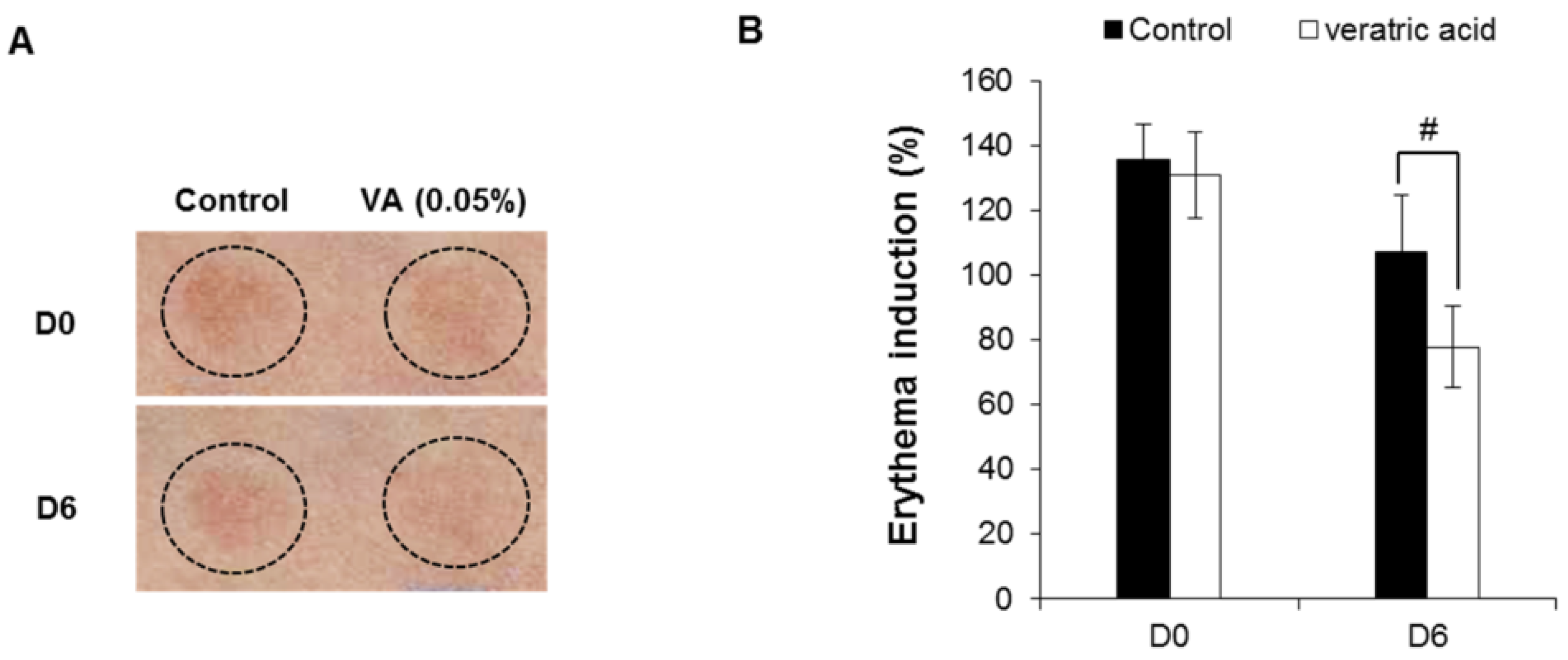
2.6. Human Skin Primary Irritation Test of Veratric Acid
| No. | Test material | 48 h | 72 h | Reaction grade b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | 1+ | 2+ | 3+ | 4+ | ± | 1+ | 2+ | 3+ | 4+ | 48 h | 72 h | Mean | ||
| 1 | Squalene | − a | − | − | − | − | − | − | − | − | − | 0 | 0 | 0 |
| 2 | VA (0.1%) | − | − | − | − | − | − | − | − | − | − | 0 | 0 | 0 |
3. Material and Methods
3.1. Chemicals and Antibodies
3.2. Cell Culture
3.3. UV Irradiation and Treatment
3.4. Cell Viability Assay
3.5. Analysis of DNA Damage by the Comet Assay
3.6. Cyclobutane Pyrimidine Dimer (CPD) Quantification
3.7. Immunofluorescence
3.8. FACS Analysis
3.9. Western Blot Analysis
3.10. Intracellular GSH Level
3.11. Inflammatory Cytokine Assay
3.12. Clinical Study of Skin Recovery Effect of Veratric Acid on UV-induced Damage Skin
3.13. Human Skin Primary Irritation Test
3.14. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocacinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef]
- Cho, J.W.; Park, K.; Kweon, G.R.; Jang, B.C.; Baek, W.K.; Suh, M.H.; Kim, C.W.; Lee, K.S.; Suh, S.I. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocyte (HaCaT) by inhibiting activation of AP-1: P38 MAP kinase and JNK as potential upstream targets. Exp. Mol. Med. 2005, 37, 186–192. [Google Scholar] [CrossRef]
- Lu, Y.P.; Lou, Y.R.; Yen, P.; Mitchell, D.; Huang, M.T.; Conney, A.H. Time course for early adaptive responses to ultraviolet B light in the epidermis of SKH-1 mice. Cancer Res. 1999, 59, 4591–4602. [Google Scholar]
- Rundhaug, J.E.; Fischer, S.M. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem. Photobiol. 2008, 84, 322–329. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Photoageing: mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar]
- Katiyar, S.K.; Mukhtar, H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001, 69, 719–726. [Google Scholar]
- Mittal, A.; Elmets, C.A.; Katiyar, S.K. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis 2003, 24, 1379–1388. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar]
- Staniforth, V.; Huang, W.C.; Aravindaram, K.; Yang, N.S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Robberecht, R.; Flint, S.D. Internal filters: Prospects for UV-acclimation in higher plants. Physiol. Plant 1983, 58, 445–450. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef]
- Jedinak, A.; Dudhgaonkar, S.; Wu, Q.L.; Simon, J.; Sliva, D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011, 10, 52. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Park, H.G.; Kim, Y.O.; Kang, J.S.; Kim, H.M.; Hong, J.T.; Kim, Y.; Han, S.B. Induction of dendritic cell maturation by β-glucan isolated from Sparassiscrispa. Int. Immunopharmacol. 2010, 10, 1284–1294. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; Ro, H.M.; Chung, I.M. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Narasimhan, B.; Ohlan, S.; Ohlan, R.; Judge, V.; Narang, R. Hansch analysis of veratric acid derivatives as antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 689–700. [Google Scholar] [CrossRef]
- Saravanakumar, M.; Raja, B. Veratric acid, a phenolic acid attenuates blood pressure and oxidative stress in L-NAME induced hypertensive rats. Eur. J. Pharmacol. 2011, 671, 87–94. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Creagh, E.M.; Martin, S.J. Capases: cellular demolition experts. Biochem. Soc. Trans. 2001, 29, 696–702. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Tripp, C.S.; Blomme, E.A.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J. Invest. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef]
- Rodriguez-Burford, C.; Tu, J.H.; Mercurio, M.; Carey, D.; Han, R.; Gordon, G.; Niwas, S.; Bell, W.; Elmets, C.A.; Grizzle, W.; Pentland, A.P. Selective cyclooxygenase-2 inhibition produces heterogeneous erythema response to ultraviolet irradiation. J. Invest. Dermatol. 2005, 125, 1317–1320. [Google Scholar] [CrossRef]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006, 5, 296–308. [Google Scholar] [CrossRef]
- Image J, Java-based image processing program. National Institutes of Health: Bethesda, ML, USA, 1997–2012.
- Frosch, P.J.; Kligman, A.M. The soap chamber test. A new method for assessing the irritancyof soaps. J. Am. Acad. Dermatol. 1979, 1, 35–41. [Google Scholar] [CrossRef]
- CTFA Safety Testing Guideline; The Cosmetic, Toiletry and Fragrance Association, Inc.: Washington, DC, USA, 1981; p. 20005.
- Statview 5, a statistics application released for Apple Macintosh computers. Abacus Concepts: Piscataway, NJ, USA, 2009.
- Sample Availability: Samples of the veratric acid are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shin, S.W.; Jung, E.; Kim, S.; Lee, K.-E.; Youm, J.-K.; Park, D. Antagonist Effects of Veratric Acid against UVB-Induced Cell Damages. Molecules 2013, 18, 5405-5419. https://doi.org/10.3390/molecules18055405
Shin SW, Jung E, Kim S, Lee K-E, Youm J-K, Park D. Antagonist Effects of Veratric Acid against UVB-Induced Cell Damages. Molecules. 2013; 18(5):5405-5419. https://doi.org/10.3390/molecules18055405
Chicago/Turabian StyleShin, Seoung Woo, Eunsun Jung, Seungbeom Kim, Kyung-Eun Lee, Jong-Kyung Youm, and Deokhoon Park. 2013. "Antagonist Effects of Veratric Acid against UVB-Induced Cell Damages" Molecules 18, no. 5: 5405-5419. https://doi.org/10.3390/molecules18055405




