Hexachlorocyclotriphosphazene (HCCP)-Mediated Direct Formation of Thioethers and Ethers from Quinazolin-4(3H)-ones
Abstract
:1. Introduction
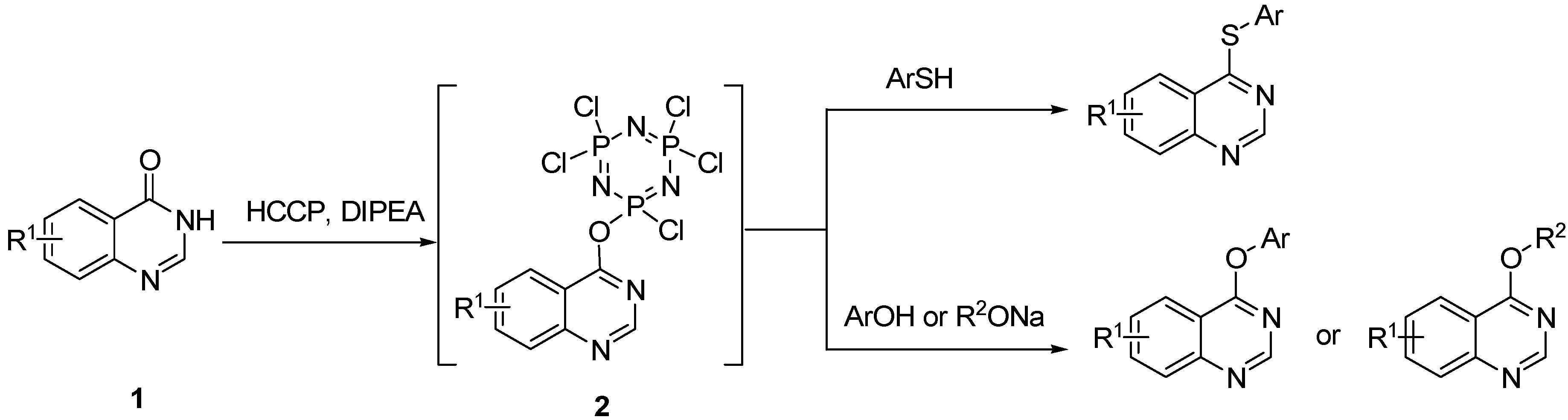
2. Results and Discussion
| Entry | Base | Solvent | Temperature (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | TEA | MeCN | 45 | 48 |
| 2 | K2CO3 | MeCN | 45 | 32 |
| 3 | Cs2CO3 | MeCN | 45 | 69 |
| 4 | DIPEA | MeCN | 45 | 81 |
| 5 | DIPEA | THF | 45 | 12 |
| 6 | DIPEA | DMF | 45 | 23 |
| 7 | DIPEA | CH2Cl2 | 45 | 7 |
| 8 | DIPEA | MeCN | 45 | 76 c |
| 9 | DIPEA | MeCN | 25 | 68 |
| 10 | DIPEA | MeCN | 65 | 55 |
| 11 | DIPEA | MeCN | 45 | 79 d |
| 12 | DIPEA | MeCN | 45 | 71 e |
| 13 | DIPEA | MeCN | 45 | 81 f |
| 14 | DIPEA | MeCN | 45 | 70 g |
| Entry | Quinazolin-4(3H)-one | ArSH | Product | Yield (%) b | ||
|---|---|---|---|---|---|---|
| 1 |  | 1a |  |  | 3 | 79 |
| 2 | 1a |  |  | 4 | 64 c | |
| 3 | 1a |  |  | 5 | 69 | |
| 4 | 1a |  |  | 6 | 59 | |
| 5 | 1a |  |  | 7 | 50 | |
| 6 | 1a |  |  | 8 | 86 | |
| 7 | 1a |  |  | 9 | 94 | |
| 8 | 1a |  |  | 10 | 66 | |
| 9 | 1a |  |  | 11 | 91 | |
| 10 |  | 1b |  |  | 12 | 54 d |
| 11 |  | 1c |  |  | 13 | 60 |
| 12 |  | 1d |  |  | 14 | 64 |
| 13 |  | 1e |  |  | 15 | 51 d |
| 14 |  | 1f |  |  | 16 | 79 |
| 15 |  | 1g |  |  | 17 | 72 |
| Entry | Quinazolin-4(3H)-one | ArOH or RONa | Product | Yield (%) b | |
|---|---|---|---|---|---|
| 1 | 1a |  |  | 18 | 75 |
| 2 | 1a |  |  | 19 | 52 |
| 3 | 1a |  |  | 20 | 73 |
| 4 | 1a |  |  | 21 | 51 |
| 5 | 1b |  |  | 22 | 53 c |
| 6 | 1f |  |  | 23 | 70 |
| 7 | 1g |  |  | 24 | 70 |
| 8 | 1a | CH3CH2ONa |  | 25 | 54 |
| 9 | 1b | CH3CH2ONa |  | 26 | 33 c |
| 10 | 1d | CH3CH2ONa |  | 27 | 67 |
| 11 | 1g | CH3CH2ONa |  | 28 | 64 |
| 12 | 1a | CH3CH2CH2ONa |  | 29 | 48 |
3. Experimental
3.1. General
3.2. General Procedure for HCCP-Mediated Formation of Thioethers and Ethers from Quinazolin-4(3H)-ones
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hori, M.; Iemura, R.; Hara, H.; Ozaki, A.; Sukamoto, T.; Ohtaka, H. Novel 4-phenoxy-2-(1-piperazinyl)quinazolines as potent anticonvulsive and antihypoxic agents. Chem. Pharm. Bull. 1990, 38, 681–687. [Google Scholar] [CrossRef]
- Havera, H.J.; Vidrio, H.J. Derivatives of 1,3-disubstituted 2,4(1H,3H)-quinazolinediones as possible peripheral vasodilators or antihypertensive agents. J. Med. Chem. 1979, 22, 1548–1550. [Google Scholar]
- Chao, Q.; Deng, L.; Shih, H.; Leoni, L.M.; Genini, D.; Carson, D.A.; Cottam, H.B. Substituted isoquinolines and quinazolines as potential antiinflammatory agents. Synthesis and biological evaluation of inhibitors of tumor necrosis factor α. J. Med. Chem. 1999, 42, 3860–3873. [Google Scholar]
- Kung, P.P.; Casper, M.D.; Cook, K.L.; Wilson-Lingardo, L.; Risen, L.M.; Vickers, T.A.; Ranken, R.; Blyn, L.B.; Wyatt, J.R.; Cook, P.D.; et al. Structure−activity relationships of novel 2-substituted quinazoline antibacterial agents. J. Med. Chem. 1999, 42, 4705–4713. [Google Scholar]
- Liverton, N.J.; Armstrong, D.J.; Claremon, D.A.; Remy, D.C.; Bardwin, J.J.; Lynch, R.J.; Zhang, G.; Gould, R.J. Nonpeptide glycoprotein IIb/IIIa inhibitors: Substituted quinazolinediones and quinazolinones as potent fibrinogen receptor antagonists. Bioorg. Med. Chem. Lett. 1998, 8, 483–486. [Google Scholar]
- Brunton, S.A.; Stibbard, J.H.A.; Rubin, L.L.; Kruse, L.I.; Guicherit, O.M.; Boyd, E.A.; Price, S. Potent inhibitors of the Hedgehog signaling pathway. J. Med. Chem. 2008, 51, 1108–1110. [Google Scholar]
- Smits, R.A.; de Esch, I.J.P.; Zuiderveld, O.P.; Broeker, J.; Sansuk, K.; Guaita, E.; Coruzzi, G.; Adami, M.; Haaksma, E.; Leurs, R. Discovery of quinazolines as histamine H4 receptor inverse agonists using a scaffold hopping approach. J. Med. Chem. 2008, 51, 7855–7865. [Google Scholar] [CrossRef]
- Sirisoma, N; Pervin, A.; Zhang, H.; Jiang, S.C.; Willardsen, J.A.; Anderson, M.B.; Mather, G.; Pleiman, C.M.; Kasibhatla, S.; Tseng, B.; et al. Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine, a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration. J. Med. Chem. 2009, 52, 2341–2351. [Google Scholar] [CrossRef]
- Zeng, Z.-S.; He, Q.-Q.; Liang, Y.-H.; Feng, X.-Q.; Chen, F.-E.; Clercq, E.D.; Balzarini, J. Hybrid diarylbenzopyrimidine non-nucleoside reverse transcriptase inhibitors as promising new leads for improved anti-HIV-1 chemotherapy. Bioorg. Med. Chem. 2010, 18, 5039–5047. [Google Scholar]
- Nakamura, H.; Onagi, S. Synthesis and biological evaluation of allenic quinazolines using palladium-catalyzed hydride-transfer reaction. Tetrahedron Lett. 2006, 47, 2539–2542. [Google Scholar] [CrossRef]
- Castera-Ducros, C.; Azas, N.; Verhaeghe, P.; Hutter, S.; Garrigue, P.; Dumetre, A.; Mbatchi, L.; Laget, M.; Remusat, V.; Sifredi, F.; et al. Targeting the human malaria parasite Plasmodium falciparum: In vitro identification of a new antiplasmodial hit in 4-phenoxy-2-trichloromethylquinazoline series. Eur. J. Med. Chem. 2011, 46, 4184–4191. [Google Scholar] [CrossRef]
- Holladay, M.W.; Campbell, B.T.; Rowbottom, M.W.; Chao, Q.; Sprankle, K.G.; Lai, A.G.; Abraham, S.; Setti, E.; Faraoni, R.; Tran, L.; et al. 4-Quinazolinyloxy-diaryl ureas as novel BRAFV600E inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5342–5346. [Google Scholar] [CrossRef]
- Rowbottom, M.W.; Faraoni, R.; Chao, Q.; Campbell, B.T.; Lai, A.G.; Setti, E.; Ezawa, M.; Sprankle, K.G.; Abraham, S.; Tran, L.; et al. Identification of 1-(3-(6,7-dimethoxyquinazolin-4-yloxy)phenyl)-3-(5-(1,1,1-trifluoro-2-methylpropan-2-yl)isoxazol-3-yl)urea hydrochloride (CEP-32496), a highly potent and orally efficacious inhibitor of V-RAF murine sarcoma viral oncogene homologue B1 (BRAF) V600E. J. Med. Chem. 2012, 55, 1082–1105. [Google Scholar] [CrossRef]
- Ple, P.A.; Jung, F.; Ashton, S.; Hennequin, L.; Laine, R.; Morgentin, R.; Pasquet, G.; Taylor, S. Discovery of AZD2932, a new Quinazoline Ether Inhibitor with high affinity for VEGFR-2 and PDGFR tyrosine kinases. Bioorg.Med. Chem. Lett. 2012, 22, 262–266. [Google Scholar]
- Yang, S.; Li, Z.; Jin, L.H.; Song, B.A.; Liu, G.; Chen, J.; Chen, Z.; Hu, D.Y.; Xue, W.; Xu, R.Q. Synthesis and bioactivity of 4-alkyl(aryl)thioquinazoline derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 2193–2196. [Google Scholar]
- Verhaeghe, P.; Dumetre, A.; Castera-Ducros, C.; Hutter, S.; Laget, M.; Fersing, C.; Prieri, M.; Yzombard, J.; Sifredi, F.; Rault, S.; et al. 4-Thiophenoxy-2-trichloromethyquinazolines display in vitro selective antiplasmodial activity against the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2011, 21, 6003–6006. [Google Scholar] [CrossRef]
- Garofalo, A.; Goossens, L.; Baldeyrou, B.; Lemoine, A.; Ravez, S.; Six, P.; David-Cordonnier, M.-H.; Bonte, J.-P.; Depreux, P.; Lansiaux, A.; et al. Design, synthesis, and DNA-binding of N-alkyl(anilino)quinazoline derivatives. J. Med. Chem. 2010, 53, 8089–8103. [Google Scholar]
- Harris, C.S.; Hennequin, L.F.; Willerval, O. Three-point variation of a gefinitib quinazoline core. Tetrahedron Lett. 2009, 50, 1600–1602. [Google Scholar] [CrossRef]
- Sreedhar, B.; Reddy, P.S.; Reddy, M.A. Catalyst-free and base-free water-promoted SNAr reaction of heteroaryl halides with thiols. Synthesis 2009, 1732–1738. [Google Scholar] [CrossRef]
- Wan, Z.K.; Wacharasindhu, S.; Binnun, E.; Mansour, T. An efficient direct amination of cyclic amides and cyclic ureas. Org. Lett. 2006, 8, 2425–2428. [Google Scholar]
- Wan, Z.K.; Wacharasindhu, S.; Levins, C.G.; Lin, M.; Tabei, K.; Mansour, T.S. The scope and mechanism of phosphonium-mediated SNAr reactions in heterocyclic amidesand ureas. J. Org. Chem. 2007, 72, 10194–10210. [Google Scholar] [CrossRef]
- Shen, Z.L.; Hong, Y.M.; He, X.F.; Mo, W.M.; Hu, B.X.; Sun, N.; Hu, X.Q. Switching the chemoselectivity in the amination of 4-chloroquinazolines with aminopyrazoles. Org. Lett. 2010, 12, 552–555. [Google Scholar]
- Shen, Z.L.; He, X.F.; Hong, Y.M.; Hu, X.Q.; Mo, W.M.; Hu, B.X.; Sun, N. One-pot synthesis of 4-aminoquinazolines by hexamethyldisilazane-mediated reaction of quinazolin-4(3H)-ones with amines. Synth. Commun. 2011, 41, 3644–3653. [Google Scholar] [CrossRef]
- Shen, Z.L.; He, X.F.; Dai, J.L.; Mo, W.M.; Hu, B.X.; Sun, N.; Hu, X.Q. An efficient HCCP-mediated direct amination of quinazolin-4(3H)-ones. Tetrahedron 2011, 67, 1665–1672. [Google Scholar]
- Sample Availability: Samples of the compounds 3-17 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, B.; Zhang, X.; Sheng, L.; Guo, M.; Shen, Z.; Hu, X.; Sun, N.; Mo, W. Hexachlorocyclotriphosphazene (HCCP)-Mediated Direct Formation of Thioethers and Ethers from Quinazolin-4(3H)-ones. Molecules 2013, 18, 5580-5593. https://doi.org/10.3390/molecules18055580
Hu B, Zhang X, Sheng L, Guo M, Shen Z, Hu X, Sun N, Mo W. Hexachlorocyclotriphosphazene (HCCP)-Mediated Direct Formation of Thioethers and Ethers from Quinazolin-4(3H)-ones. Molecules. 2013; 18(5):5580-5593. https://doi.org/10.3390/molecules18055580
Chicago/Turabian StyleHu, Baoxiang, Xiaochu Zhang, Lili Sheng, Ming Guo, Zhenlu Shen, Xinquan Hu, Nan Sun, and Weimin Mo. 2013. "Hexachlorocyclotriphosphazene (HCCP)-Mediated Direct Formation of Thioethers and Ethers from Quinazolin-4(3H)-ones" Molecules 18, no. 5: 5580-5593. https://doi.org/10.3390/molecules18055580







