Photophysical Studies of a New Water Soluble Indocarbocyanine Dye Adsorbed onto Microcrystalline Cellulose and beta-Cyclodextrin
Abstract
:1. Introduction
2. Results and Discussion
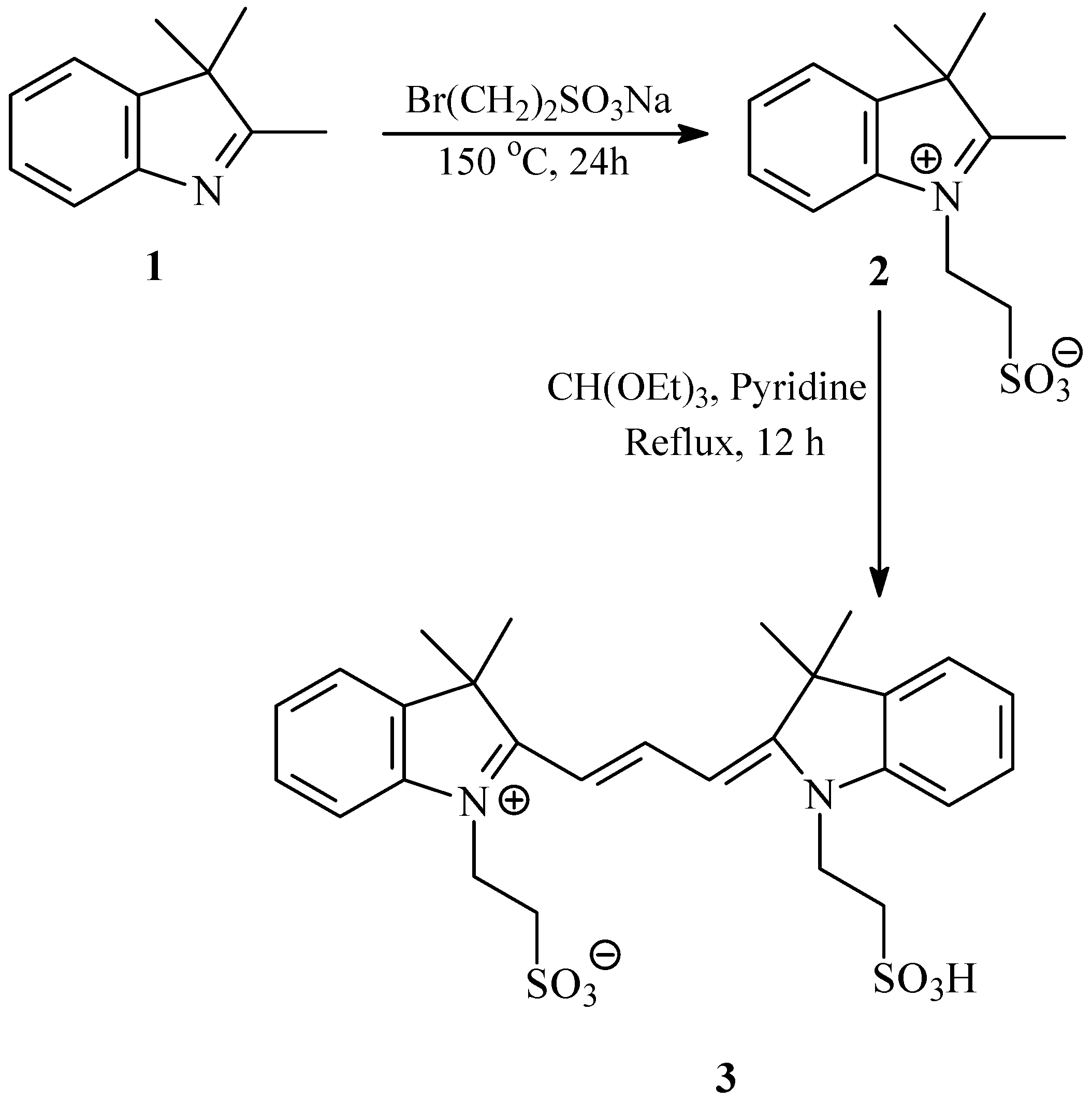
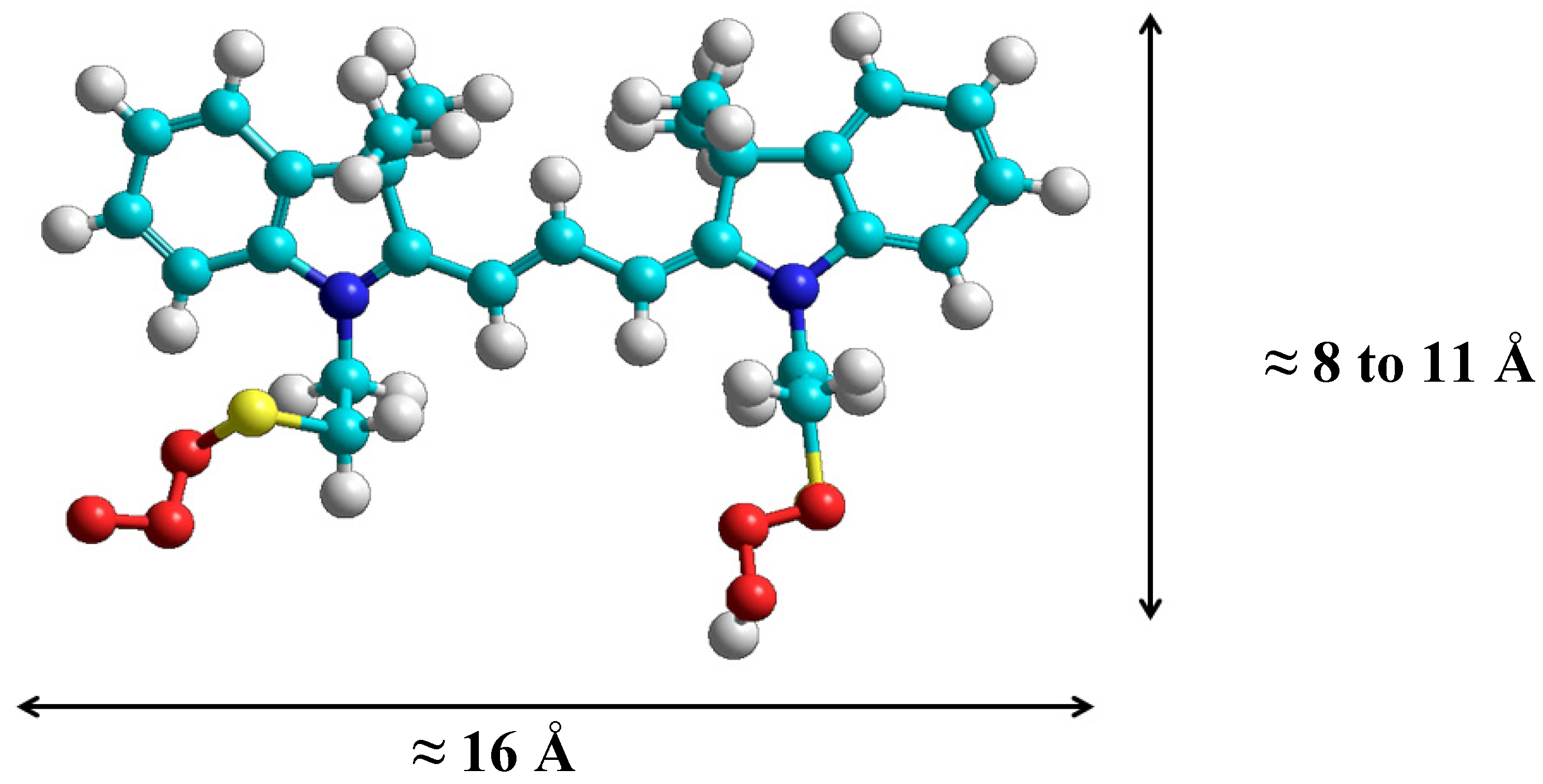
2.1. Synthesis and Spectral Properties of 2-[3-(3,3-Dimethyl-1-(2-sulfoethyl)indolin-2-ylidene)prop-1-enyl]-3,3-dimethyl-1-(2-sulfoethyl)-3H-indolium (3) in Methanol
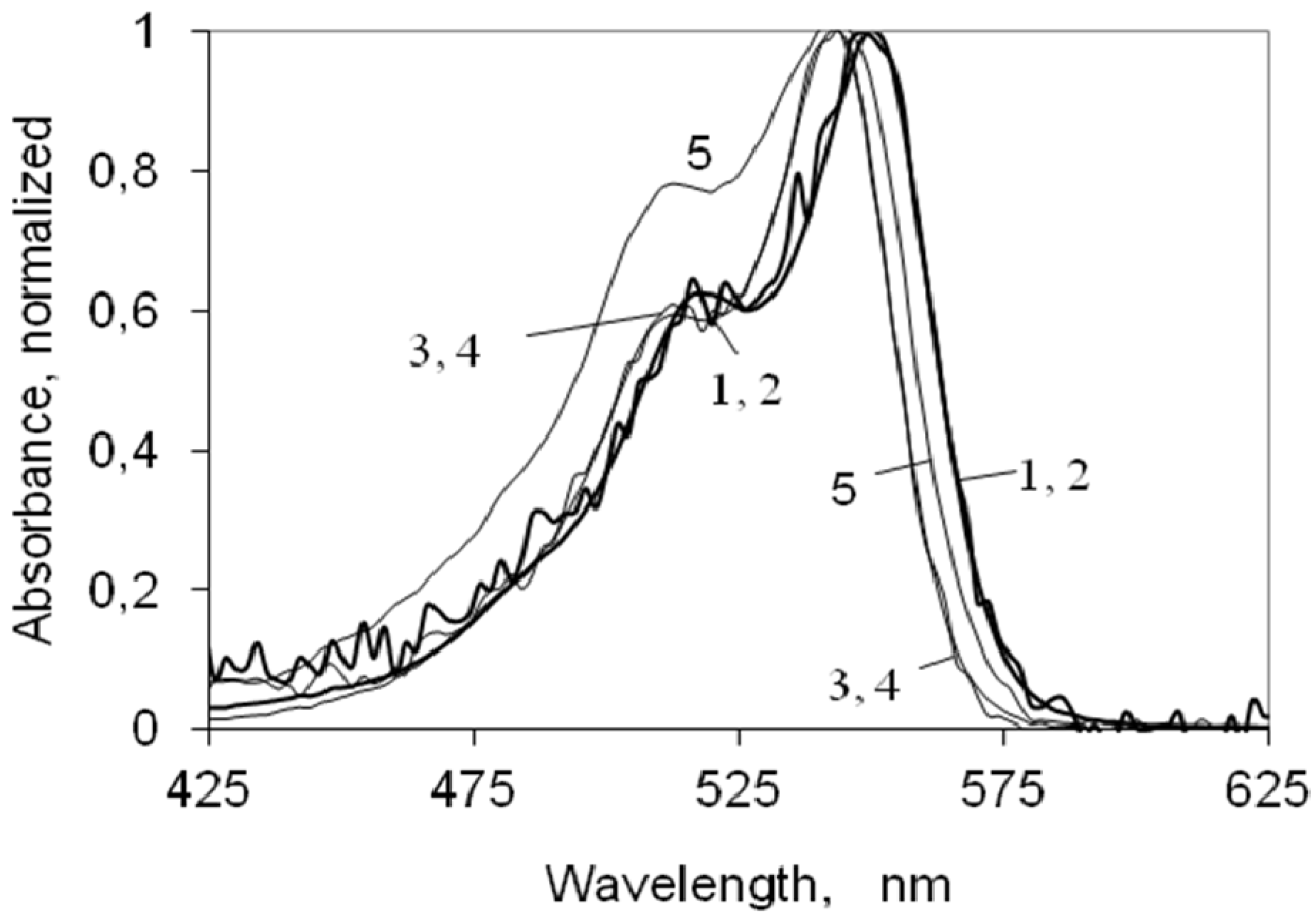
2.2. UV-Vis Ground State Absorption of the Indocarbocyanine Dye in Solution
2.3. UV-Vis Ground State Diffuse Reflectance of Powdered Solid Samples
2.3.1. Indocarbocyanine Adsorbed on Microcrystalline Cellulose
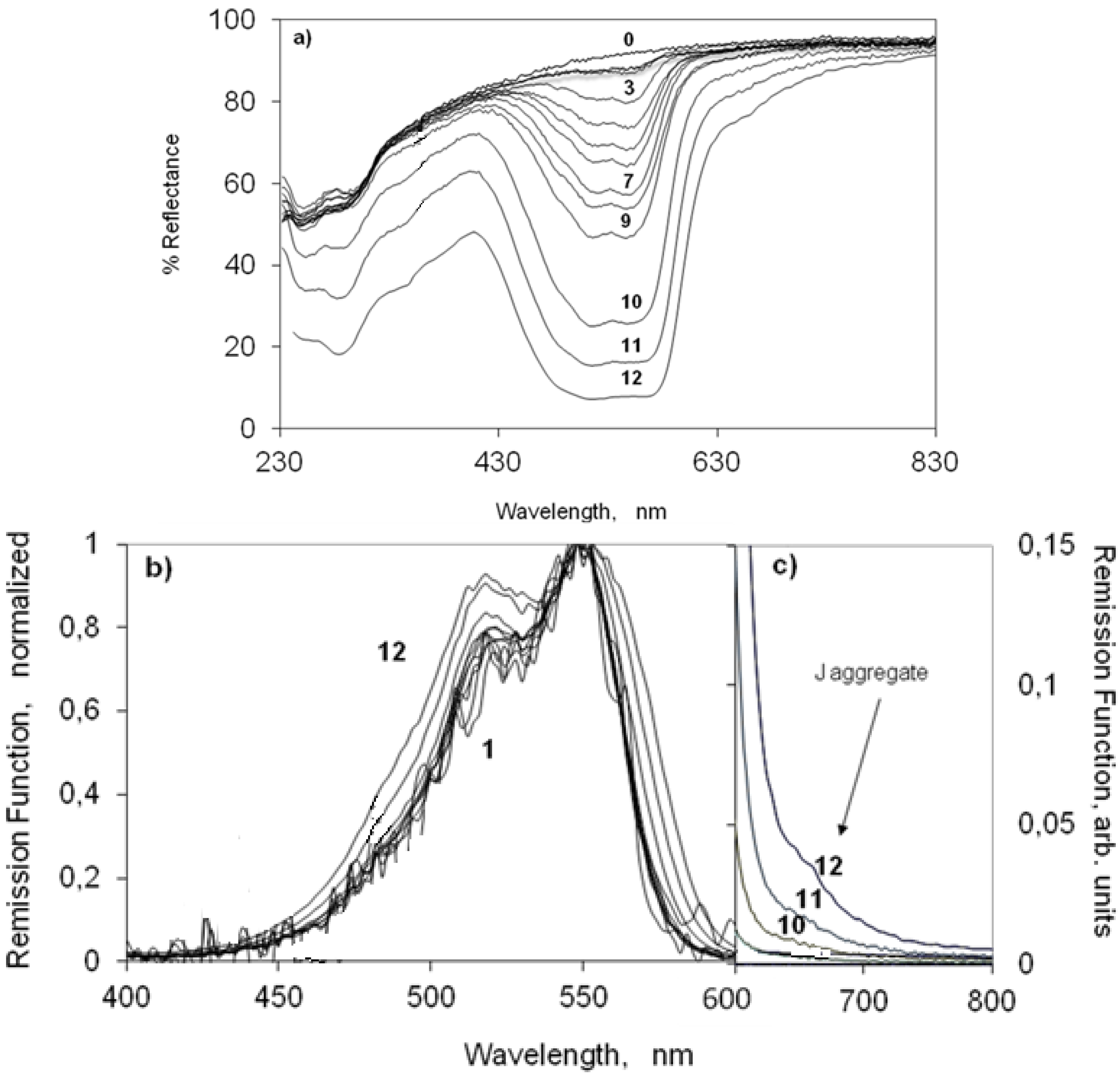
2.3.2. Indocarbocyanine on β-Cyclodextrin Solid Complexes
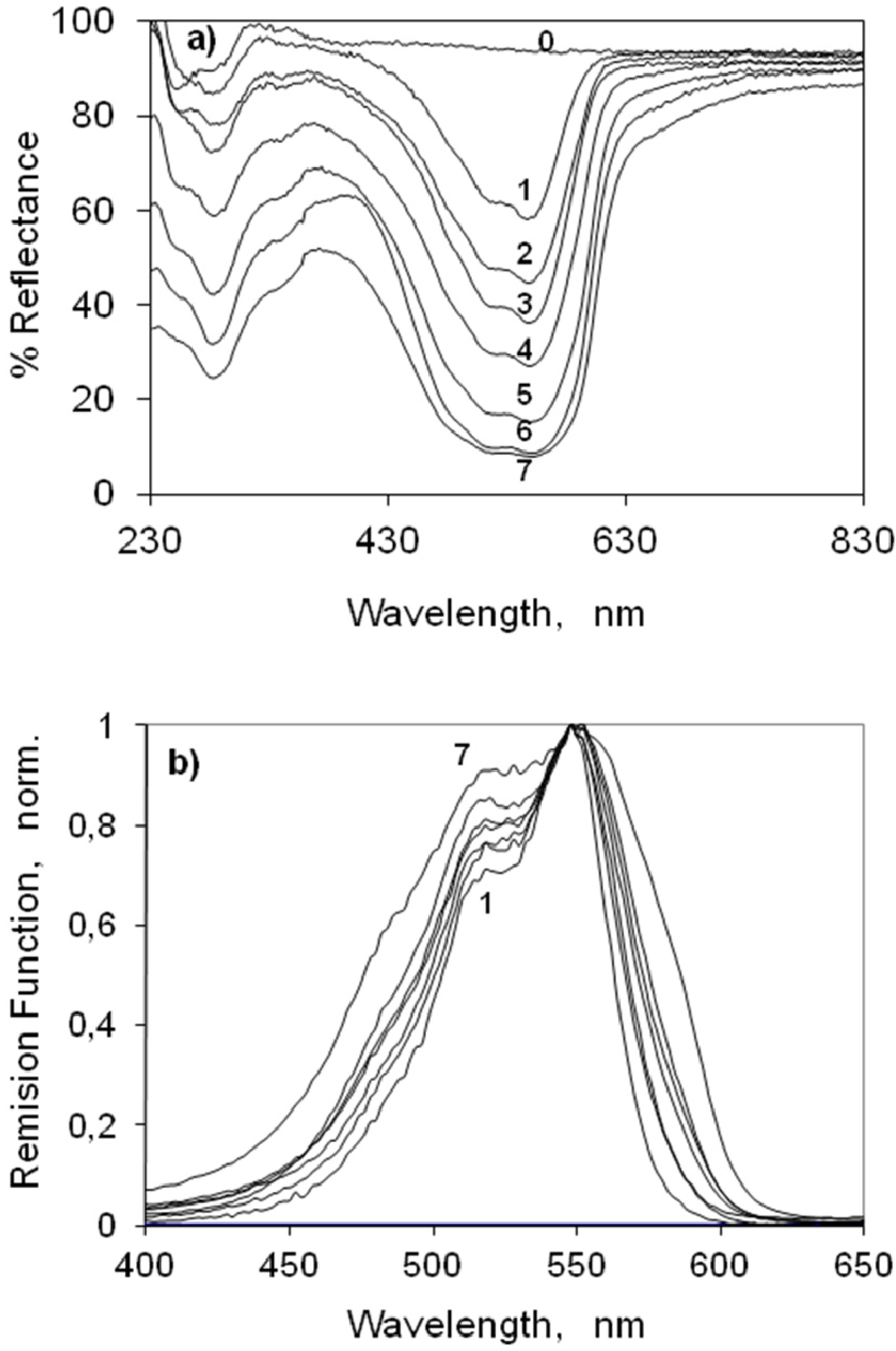
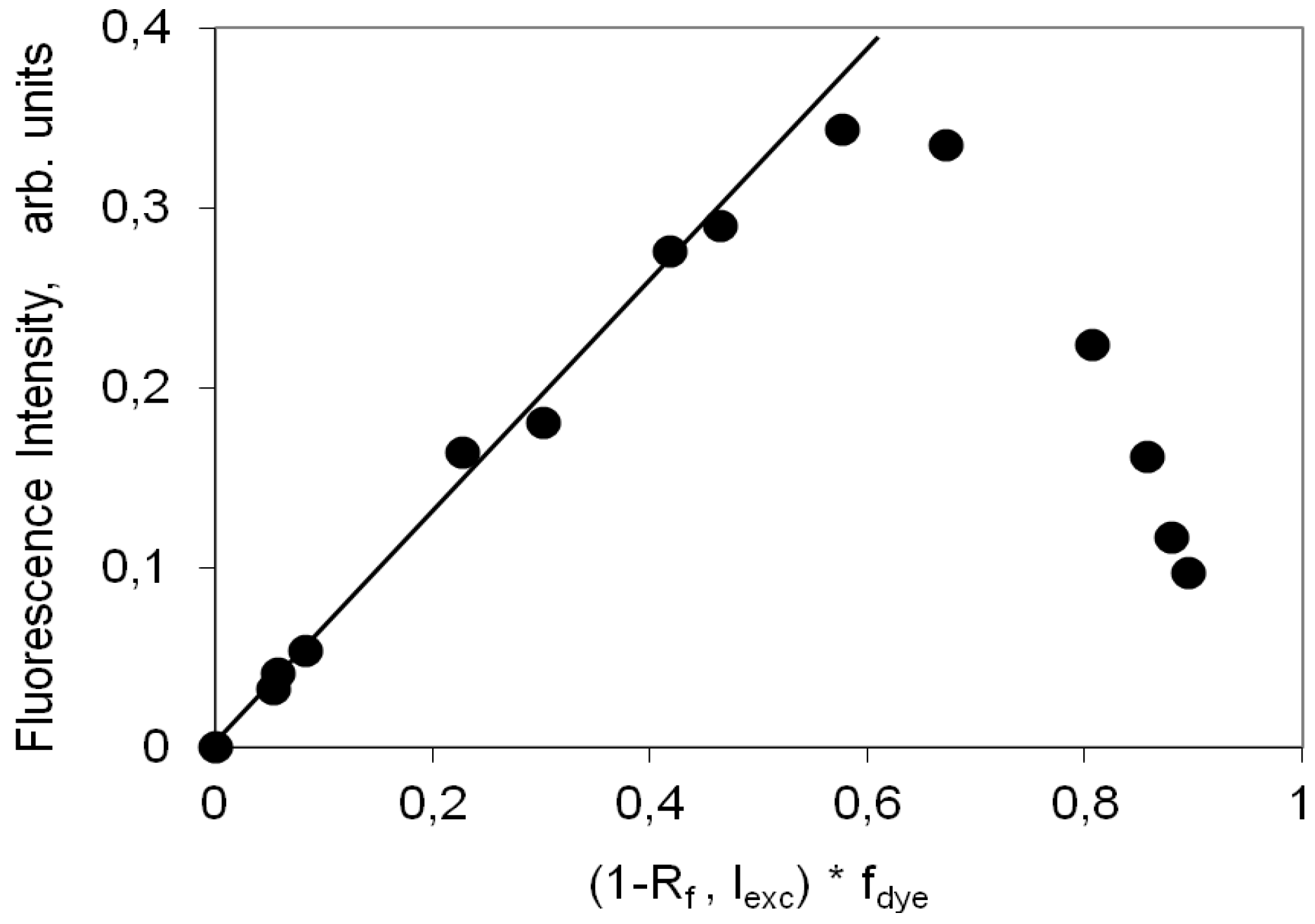
2.4. Fluorescence Spectra and Fluorescence Quantum Yield
2.4.1. Indocarbocyanine in Solution
2.4.2. Indocarbocyanine on Microcrystalline Cellulose
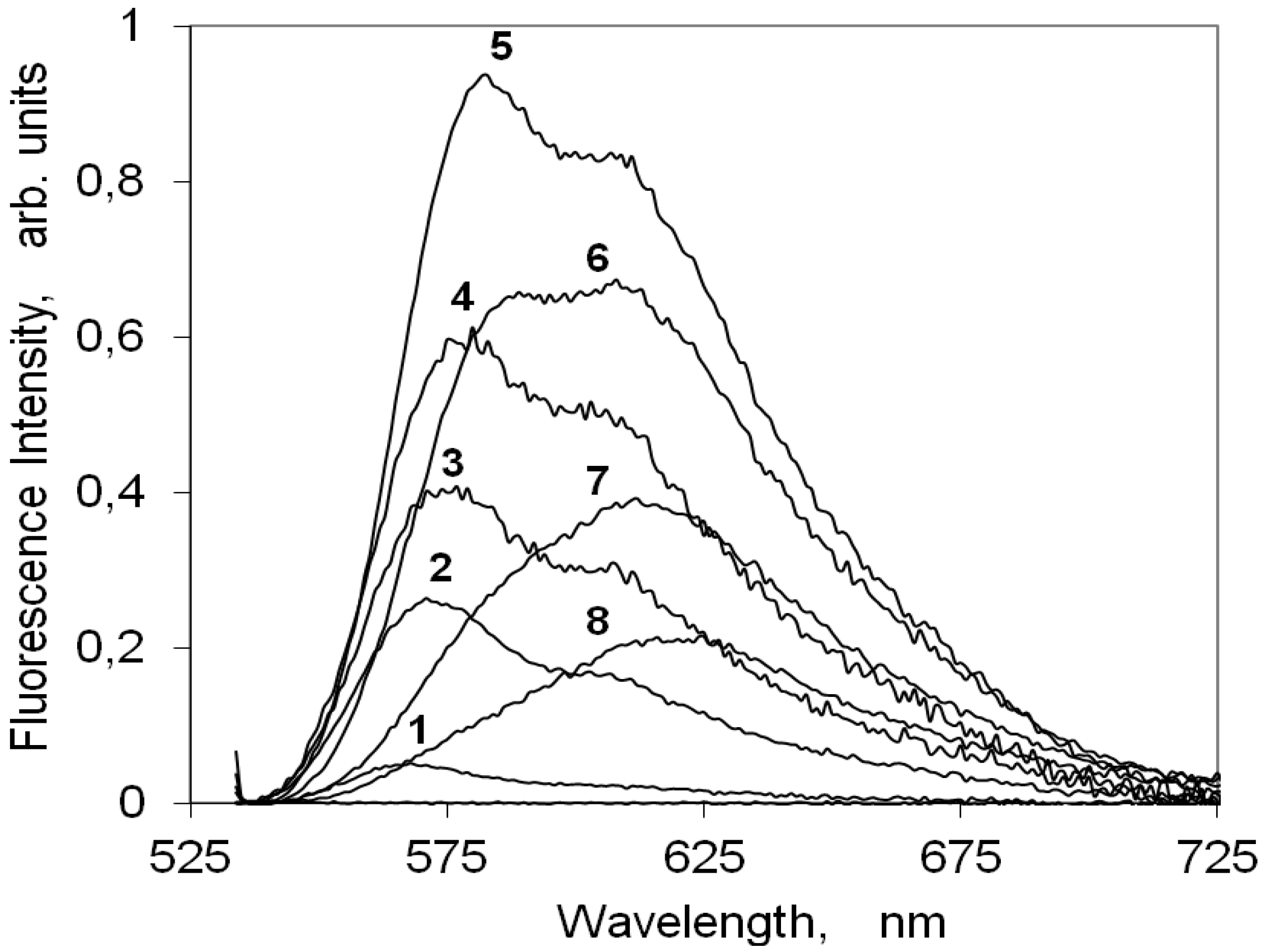
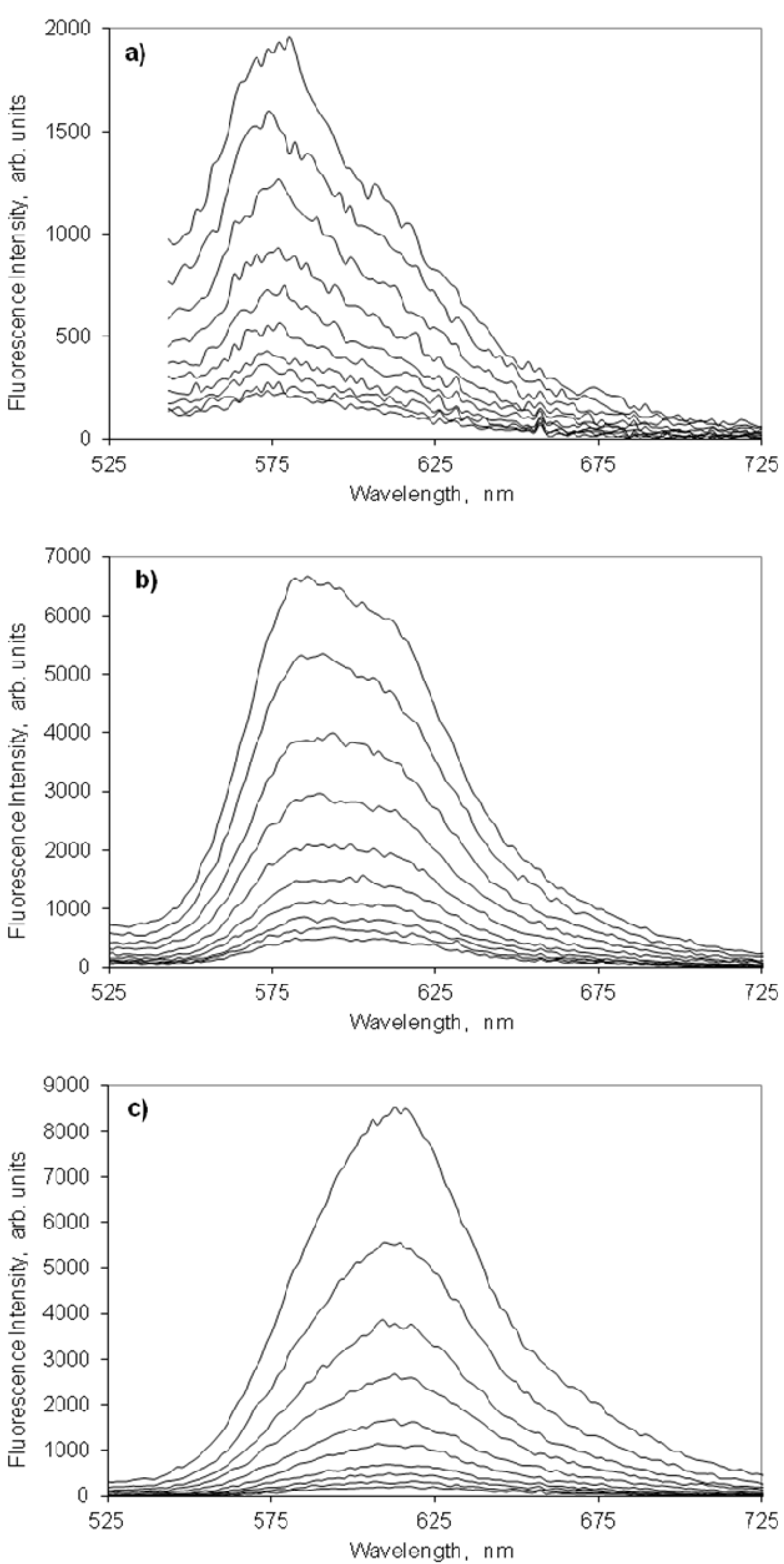
2.4.3. Indocarbocyanine on β-Cyclodextrin Solid Complexes
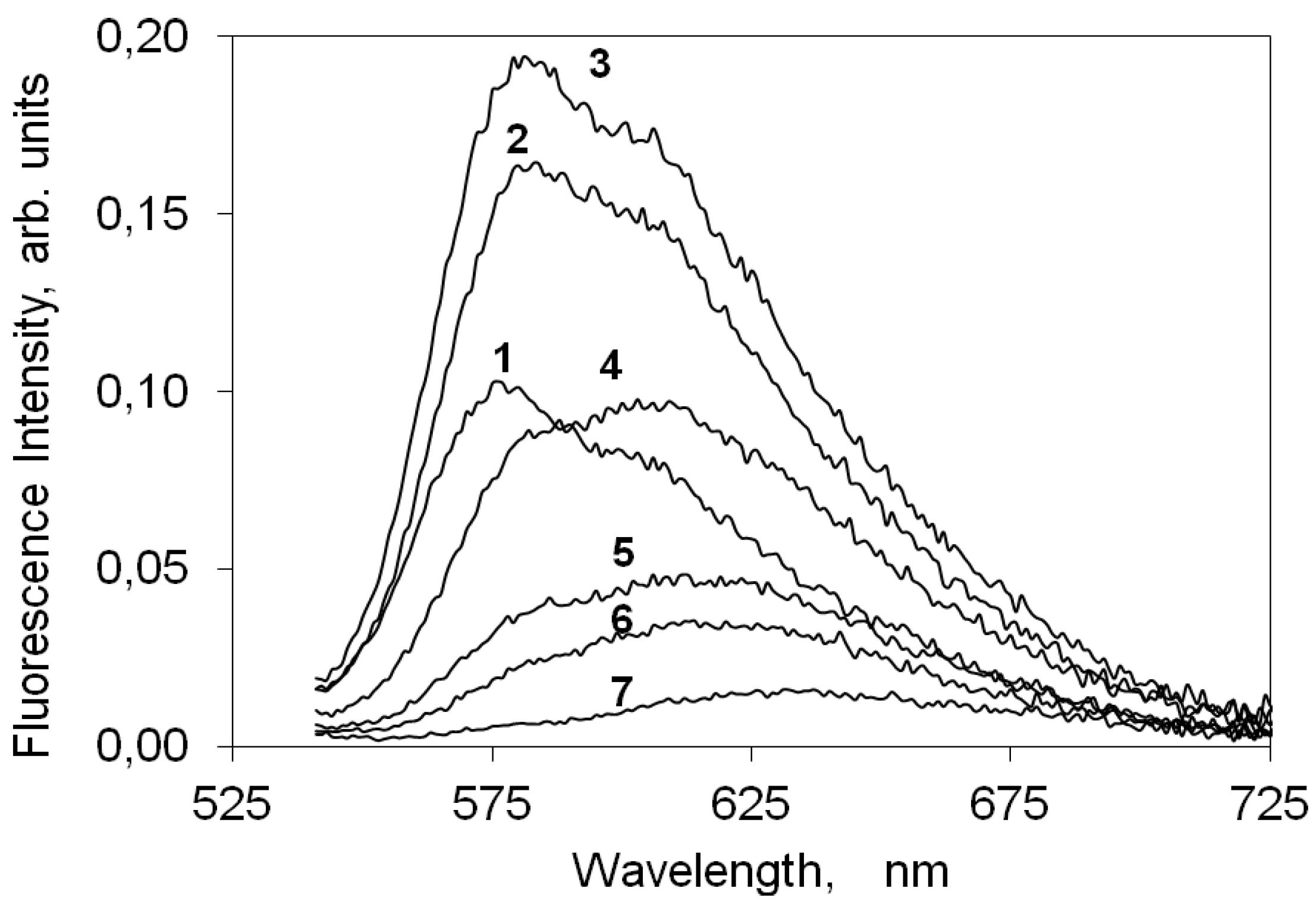
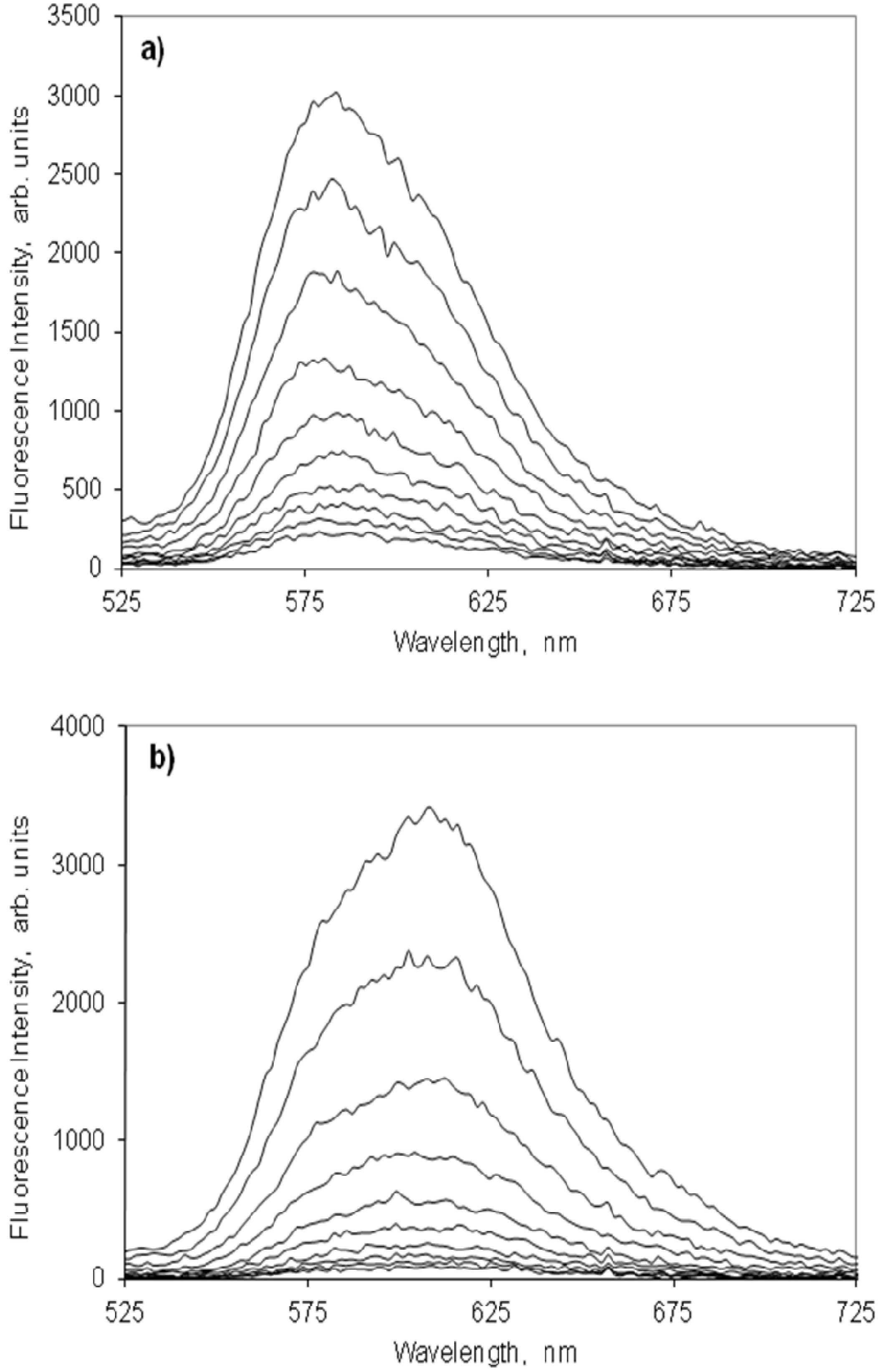
2.5. Fluorescence Lifetime and Lifetime Distribution Analysis
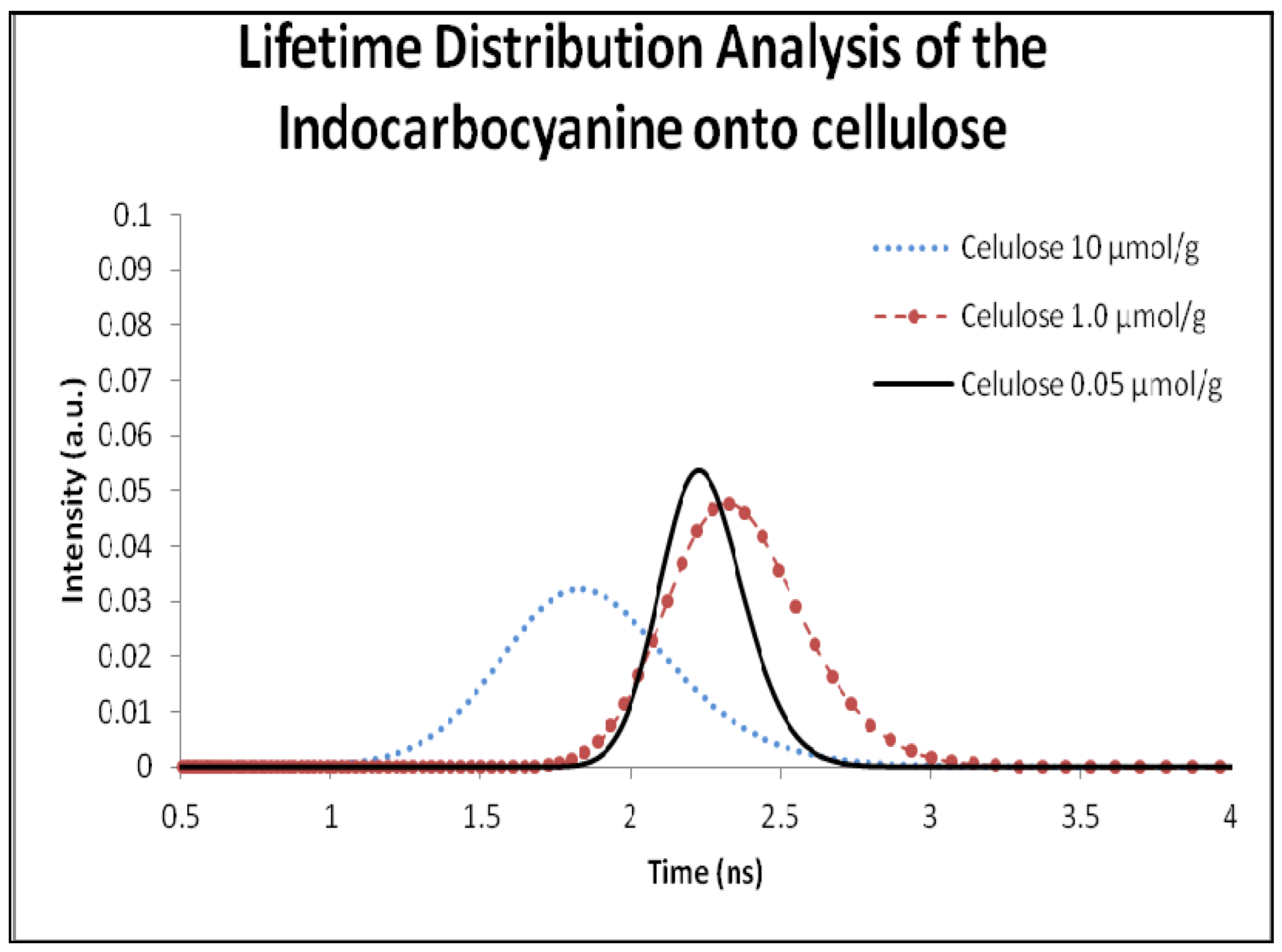
| Cellulose | β-Cyclodextrin | ||||||
|---|---|---|---|---|---|---|---|
| Concentration | Fluorescence Lifetime (ns) | Χ2 | MonoExp. Decay (ns) | Concentration | Fluorescence Lifetime (ns) | Χ2 | MonoExp. Decay (ns) |
| 10 µmol/g | 1.8 | 0.78 | 2.0 | 1:50 | 1.4 | 0.65 | 1.6 |
| 1.0 µmol | 2.3 | 0.65 | 2.5 | 1:250 | 1.7 | 0.73 | 2.1 |
| 0.5 µmol/g | 2.4 | 0.51 | 2.7 | 1:500 | 2.3 | 0.93 | 2.6 |
| 0.05 µmol/g | 2.2 | 0.74 | 2.4 | 1:1000 | 2.0 | 0.87 | 2.3 |

3. Experimental
3.1. Materials and Characterization
3.2. Synthesis of 2-[3-(3,3-Dimethyl-1-(2-sulfoethyl)indolin-2-ylidene)prop-1-enyl]-3,3-dimethyl-1-(2-sulfoethyl)-3H-indolium, Inner Salt (3)
3.3. Sample Preparation
3.4. Experimental Methods
3.4.1. Ground-State Absorption in the UV-Visible
3.4.2. Laser Induced Fluorescence Emission
3.4.3. Fluorescence Lifetimes and Lifetime Distribution Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tyutyulkov, N.; Fabian, J.; Mehlhorn, A.; Dietz, F.; Tadjer, A. Polymethine Dyes, Structure and Properties; St. Kliment Ohridski University Press: Sofia, Bulgaria, 1991. [Google Scholar]
- Zollinger, H. Color Chemistry; VCH: Weinheim, Germany, 1988. [Google Scholar]
- Panighahi, M.; Dash, S.; Patel, S.; Mishra, B.K. Synthesis of cyanine dyes: A review. Tetrahedron 2012, 68, 781–805. [Google Scholar] [CrossRef]
- Sayama, K.; Tsukagoshi, S.; Mori, T.; Hara, K.; Ohga, Y.; Shinpou, A.; Abe, Y.; Suga, S.; Arakawa, H. Efficient sensitization of nanocristaline TiO2 films with cyanines and merocyanines organic dyes. Sol. Energy Mater. Sol. Cells 2003, 80, 47–71. [Google Scholar] [CrossRef]
- Fan, B.; Castro, F.A.; Heier, J.; Hany, R.; Nüesch, F. High performing doped cyanine bilayer solarcell. Org. Electron. 2010, 11, 583–588. [Google Scholar] [CrossRef]
- Hany, R.; Fan, B.; Castro, F.A.; Heier, J.; Kylberg, W.; Nüesch, F. Strategies to improve cyanine dye multi layer organic solar cells. Prog. Photovoltaics Resear. App. 2011, 19, 851–857. [Google Scholar] [CrossRef]
- Maeda, M. Laser Dyes; Academic Press: Tokyo, Japan, 1984. [Google Scholar]
- Timtcheva, I.; Maximova, V.; Deligeorgiev, T.; Zaneva, D.; Ivanov, I. New asymmetric monomethine cyanine dyes for nucleic-acid labelling: absorption and fluorescence spectral characteristics. J. Photochem. Photobiol. A Chem. 2000, 130, 7–11. [Google Scholar] [CrossRef]
- Sowell, J.; Agnew-Heard, K.A.; Mason, J.C.; Mama, C.; Strekowski, L.; Patonay, G. Use of non-covalent labeling in illustrating ligand binding to human serum albumin via affinity capillary electrophoresis with near-infrared laser induced fluorescence detection. J. Chromatogr. 2001, 755, 91–99. [Google Scholar]
- Park, J.W.; Kim, Y.; Lee, K.-J.; Kim, D.J. Novel Cyanine Dyes with Vinylsulfone Group for Labeling Biomolecules. Bioconjug. Chem. 2012, 23, 350–362. [Google Scholar] [CrossRef]
- Matsuoka, M. Infrared Absorbing Dyes; Plenum Press: New York, NY, USA, 1990. [Google Scholar]
- Law, K.Y. Organic photoconductive materials: recent trends and developments. Chem. Rev. 1993, 93, 449–486. [Google Scholar] [CrossRef]
- Ying, L.-Q.; Branchaud, B.P. Facile Synthesis of Symmetric, Monofunctional Cyanine Dyes for Imaging Applications. Bioconjug. Chem. 2011, 22, 865–869. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Guo, K.; Akers, W.; Livingston, J.; Solomon, M.; Lee, H.; Liang, K.; Agee, A.; Achilefu, S. Rational Approach To Select Small Peptide Molecular Probes Labeled with Fluorescent Cyanine Dyes for in Vivo Optical Imaging. Biochemistry 2011, 50, 2691–2700. [Google Scholar] [CrossRef]
- Kim, S.H. Functional Dyes; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Owen, D.J.; VanDerveer, D.; Schuster, G.B. Cyanine Borate Penetrated Ion Pair Structures in Solution and the Solid State: Induced Circular Dichroism. J. Am. Chem. Soc. 1998, 120, 1705–1717. [Google Scholar] [CrossRef]
- Simonsen, K.B.; Geisler, T.; Peterson, J.C.; Arentoft, J.; Sommer-Larsen, P.; Greve, D.R.; Jakobson, C.; Becher, J.; Malagoli, M.; Bredas, J.L.; Bjørnholm, T. Bis(1,3-dithiole) Polymethine Dyes for Third-Order Nonlinear Optics—Synthesis, Electronic Structure, Nonlinear Optical Properties, and Structure-Property Relations. Eur. J. Org. Chem. 1998, 1998, 2747–2757. [Google Scholar] [CrossRef]
- James, T.H. The Theory of the Photographic Processes, 4th ed.; Macmillan: New York, NY, USA, 1977. [Google Scholar]
- Chatterjee, S.; Gattschalk, P.; Davis, P.D.; Schuster, G.B. Electron-transfer reactions in cyanine borate ion pairs: photopolymerization initiators sensitive to visible light. J. Am. Chem. Soc. 1988, 110, 2326–2328. [Google Scholar] [CrossRef]
- Jadrzejewska, B.; Pietrzak, M.; Rafiaski, Z. Phenyltrialkylborates as co-initiators with cyanine dyes in visible light polymerization of acrylates. Polymer 2011, 52, 2110–2119. [Google Scholar] [CrossRef]
- Krieg, M.; Redmond, R.W. Photophysical properties of 3,3'-dialkylthiacarbocyanine dyes in homogeneous solution. Photochem. Photobiol. 1993, 57, 472–479. [Google Scholar] [CrossRef]
- Ramaiah, D.; Joy, A.; Chandrasekhar, N.; Eldho, N.V.; Das, S.; George, M.V. Halogenated Squaraine Dyes as Potential Photochemotherapeutic Agents. Synthesis and Study of Photophysical Properties and Quantum Efficiencies of Singlet Oxygen Generation. Photochem. Photobiol. 1997, 65, 783–790. [Google Scholar] [CrossRef]
- Santos, P.F.; Reis, L.V.; Duarte, I.; Serrano, J.P.; Almeida, P.; Oliveira, A.S.; Vieira Ferreira, L.F. Synthesis and Photochemical Evaluation of Iodinated Squarylium Cyanine Dyes. Helv. Chim. Acta 2005, 88, 1135–1143. [Google Scholar] [CrossRef]
- Kulbacka, J.; Pola, A.; Mosiadz, D.; Choromanska, A.; Nowak, P.; Kotulska, M.; Majkowski, M.; Hryniewicz-jankowska, A.; Purzyc, L.; Saczko, J. Cyanines as efficient photosensitizers in photodynamic reaction: photophysical properties and in vitro photodynamic activity. Biochemistry 2011, 76, 473–479. [Google Scholar]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef]
- Chibisov, A.K.; Zakharova, G.V.; Gorner, H.; Sogulyaev, Y.A.; Mushkalo, I.L.; Tolmachev, A.I. Photorelaxation processes in covalently-linked indocarbocyanine and thiacarbocyanine dyes. J. Phys. Chem. 1995, 99, 886–893. [Google Scholar]
- Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular Chemistry; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Tsien, R.; Waggoner, A.S. Handbook of Biological Confocal Microscopy; Plenum Publishing Corporation: New York, NY, USA, 1990. [Google Scholar]
- Oliveira, A.S.; Almeida, P.; Vieira Ferreira, L.F. Photophysics of Cyanines Dyes on Surfaces: Laser Induced Photoisomer Emission of 3,3'-Dialkylthiacarbocyanines Adsorbed onto Microcrystalline Cellulose. Coll. Czech. Chem. Commun. 1999, 64, 459–473. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Oliveira, A.S.; Wilkinson, F.; Worrall, D. A New Emission from Aggregates of 2,2'-Cyanine Dyes Adsorbed onto Microcrystalline Cellulose. J. Chem. Soc. Faraday Trans. 1996, 92, 1217–1225. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Vieira Ferreira, L.F.; Wilkinson, F.; Worrall, D. Photophysics of Oxacarbocyanine Dyes on Surfaces: A Re-examination of the Origins of the “New Emission” Observed with Laser Excitation and High Concentrations of Adsorbed Dyes. J. Chem. Soc. Faraday Trans. 1996, 92, 4809–4814. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Oliveira, A.S.; Khmelinskii, I.V.; Costa, S.M.B. Sensitized absorption and emission of monomer and dimer forms of acridine orange adsorbed onto microcrystalline cellulose. J. Lumin. 1996, 60-61, 485–488. [Google Scholar]
- Vieira Ferreira, L.F.; Netto-Ferreira, J.C.; Khmelinskii, I.V.; Garcia, A.R.; Costa, S.M.B. Photochemistry on Surfaces: Matrix Isolation Mechanisms for Study of Interactions of Benzophenone Adsorbed on Microcrystalline Cellulose Investigated by Diffuse Reflectance and Luminescence Techniques. Langmuir 1995, 11, 231–236. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Freixo, M.R.; Garcia, A.R.; Wilkinson, F. Photochemistry on surfaces: fluorescence emission quantum yield evaluation of dyes adsorbed on microcrystalline cellulose. J. Chem. Soc. Faraday Trans. 1992, 88, 15–22. [Google Scholar] [CrossRef]
- Wilkinson, F.; Leicester, P.; Vieira Ferreira, L.F.; Freire, V.M.M.R. Photochemisty on surfaces: Triplet-triplet energy transfer on microcrystalline cellulose studied by diffuse reflectance transient absorption and emission spectroscopy. Photochem. Photobiol. 1991, 54, 599–608. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Branco, T.J.F.; Rego, A.M.B. Luminescence quantum yields determination for molecules adsorbed onto solid powdered particles: a new method based on ground state diffuse reflectance spectra. Chem. Phys. Chem. 2004, 5, 1848–1854. [Google Scholar] [CrossRef]
- Branco, T.J.F.; Botelho do Rego, A.M.; Ferreira Machado, I.; Vieira Ferreira, L.F. A luminescence lifetime distributions analysis in heterogeneous systems by the use of Excel’s Solver. J. Phys. Chem. B 2005, 109, 15958–15967. [Google Scholar]
- Duarte, P.; Ferreira, D.P.; Ferreira Machado, I.; Vieira Ferreira, L.F.; Rodríguez, H.B.; San Román, E. Phloxine B as a probe for entrapment in Microcrystalline Cellulose. Molecules 2012, 17, 1602–1616. [Google Scholar] [CrossRef]
- Botelho do Rego, A.M.; Vieira Ferreira, L.F. Photonic and electronic spectroscopies for the characterization of organic surfaces and organic molecules adsorbed on surfaces. In Handbook of Surfaces and Interfaces of Materials; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 2, Chapter 7; pp. 275–313. [Google Scholar]
- Vieira Ferreira, L.F.; Oliveira, A.S.; Netto-Ferreira, J.C. Room temperature phosphorescence of Aromatic Ketones included into hydrophobic powdered substrates. In Fluorescence Microscopy and Fluorescent Probes 3; Kotyc, A., Ed.; Espero Publishing: Prague, Czech Republic, 1999; pp. 199–208. [Google Scholar]
- Vieira Ferreira, L.F.; Ferreira Machado, I. Surface photochemistry: Organic molecules within nanocavities of calixarenes. Curr. Drug Discov. Technol. 2007, 4, 229–245. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Ferreira, D.P.; Duarte, P.; Oliveira, A.S.; Torres, E.; Ferreira Machado, I.; Almeida, P.; Reis, L.V.; Santos, P.F. Surface Photochemistry: 3,3'-Dialkylthia and Selenocarbocyanine Dyes Adsorbed onto Microcrystalline Cellulose. Int. J. Mol. Sci. 2012, 13, 596–611. [Google Scholar] [CrossRef]
- Dorn, H.P.; Muller, A. Temperature dependence of the fluorescence lifetime and quantum yield of pseudoisocyanine monomers. Chem. Phys. Lett. 1986, 130, 426–431. [Google Scholar] [CrossRef]
- Humphry-Baker, R.; Gratzel, M.; Steiger, R. Drastic fluorescence enhancement and photochemical stabilization of cyanine dyes through micellar systems. J. Am. Chem. Soc. 1980, 102, 847–848. [Google Scholar]
- Guether, R.; Reddington, M.V. Photostable cyanine dye β-cyclodextrin conjugates. Tetrahedron Lett. 1997, 38, 6167–6170. [Google Scholar] [CrossRef]
- Battista, O.A. Microcrystalline Cellulose. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Gaylord, N.G., Bikales, N.M., Eds.; Wiley: New York, NY, USA, 1965; Volume 3, pp. 285–293. [Google Scholar]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- Chibisov, A.K.; Zakharova, G.V. Effects of substituents in the polymethine chain on the photoprocesses in indodicarbocyanine dyes. J. Chem. Faraday Trans. 1996, 92, 4917–4925. [Google Scholar]
- Chen, P.; Sun, S.; Hu, Y.; Qian, Z.; Zheng, D. Structure and solvent effect on the photostability of indolenine cyanine dyes. Dyes Pigments 1999, 41, 227–231. [Google Scholar] [CrossRef]
- Dai, Z.; Peng, B.; Chen, X. Crystal structure of 10-chloro-3,3,3′,3′-tetramethyl-1,1′-diethylindodicarbocyanine. Dyes Pigments 1999, 40, 219–223. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M. Functional Dyes, and Some Hi-Tech Applications. Int. J. Photoen. 2009, 2009, 434897. [Google Scholar]
- Song, F.; Peng, X.; Lu, E.; Zhang, R.; Chen, X.; Song, B. Syntheses, spectral properties and photostabilities of novel water-soluble near-infrared cyanine dyes. J. Photochem. Photobiol. A Chem. 2004, 168, 53–57. [Google Scholar]
- Hamer, F.M. The cyanine dyes and related compounds. In The Chemistry of Heterocyclic Compounds; Weissberger, A., Ed.; Wiley-Interscience: New York, NY, USA, 1964; Volume 18. [Google Scholar]
- El-Shishtawy, R.M.; Almeida, P. A New Vilsmeier-type Reaction for One-pot Synthesis of pH Sensitive Fluorescent Cyanine Dyes. Tetrahedron 2006, 62, 7793–7798. [Google Scholar] [CrossRef]
- PhotochemCAD. Available online: Available online: http://omlc.ogi.edu/spectra/PhotochemCAD/html/016.html/ (accessed on 15 January 2013).
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El-Shishtawy, R.M.; Oliveira, A.S.; Almeida, P.; Ferreira, D.P.; Conceição, D.S.; Ferreira, L.F.V. Photophysical Studies of a New Water Soluble Indocarbocyanine Dye Adsorbed onto Microcrystalline Cellulose and beta-Cyclodextrin. Molecules 2013, 18, 5648-5668. https://doi.org/10.3390/molecules18055648
El-Shishtawy RM, Oliveira AS, Almeida P, Ferreira DP, Conceição DS, Ferreira LFV. Photophysical Studies of a New Water Soluble Indocarbocyanine Dye Adsorbed onto Microcrystalline Cellulose and beta-Cyclodextrin. Molecules. 2013; 18(5):5648-5668. https://doi.org/10.3390/molecules18055648
Chicago/Turabian StyleEl-Shishtawy, Reda M., Anabela S. Oliveira, Paulo Almeida, Diana P. Ferreira, David S. Conceição, and Luis F. Vieira Ferreira. 2013. "Photophysical Studies of a New Water Soluble Indocarbocyanine Dye Adsorbed onto Microcrystalline Cellulose and beta-Cyclodextrin" Molecules 18, no. 5: 5648-5668. https://doi.org/10.3390/molecules18055648