Vasodilator Compounds Derived from Plants and Their Mechanisms of Action
Abstract
:1. Introduction
2. Search Strategy
3. Types of Compounds with Vasodilator Effects

4. Proposed Mechanisms of Action
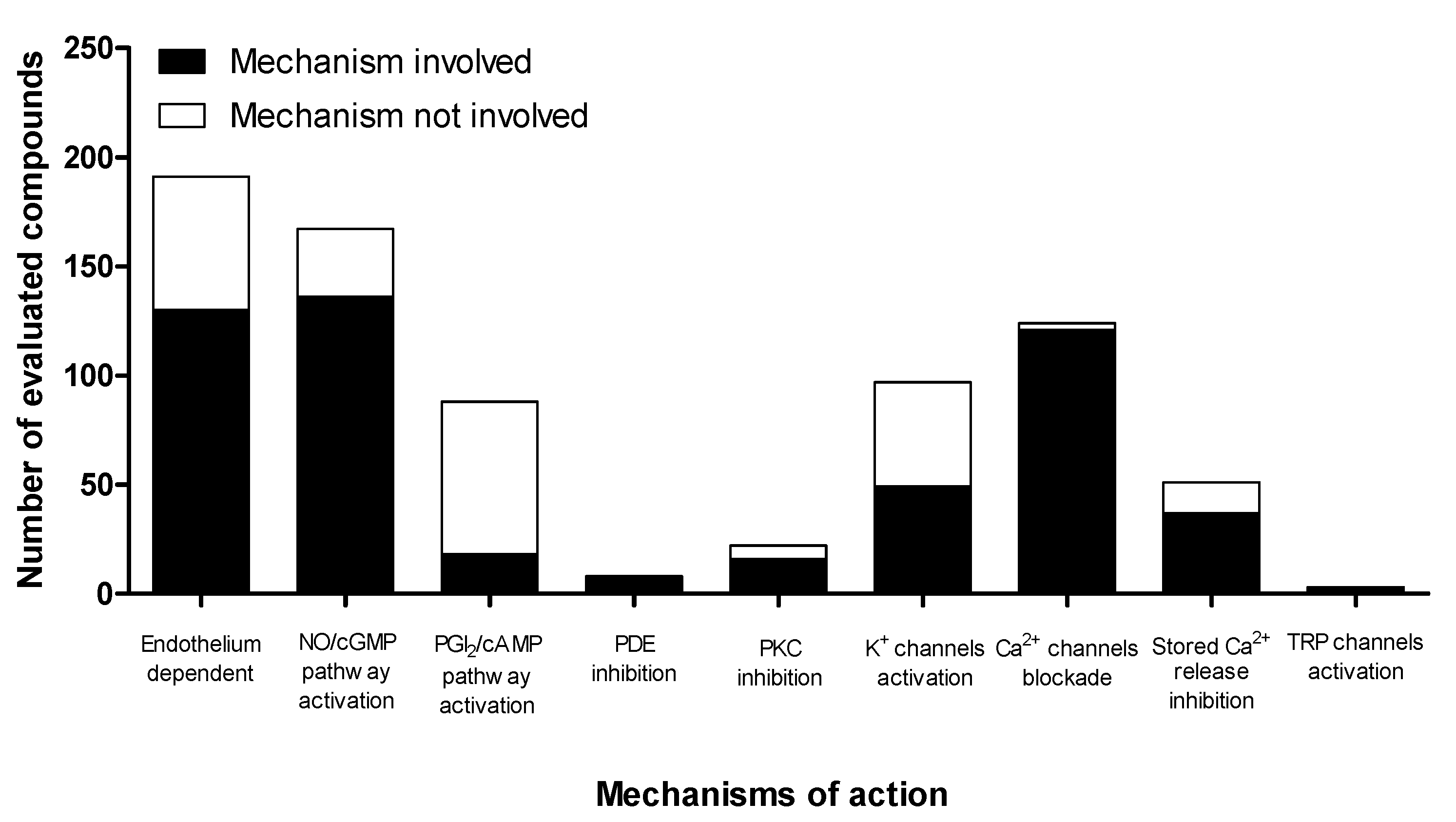
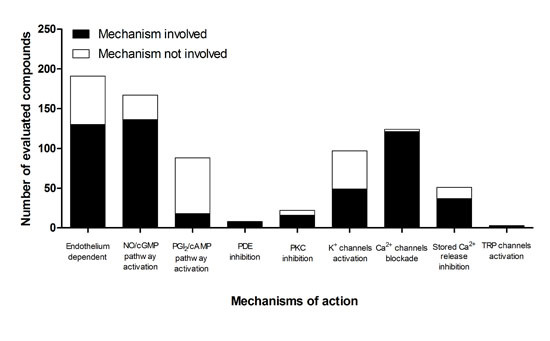
5. Participation of the Endothelium in the Mechanism of Action
| Compound | Type of artery/vein | EC50 | Endothelium | NO/cGMP | PGI2/cAMP | PDE | PKC | K+ Ch | Ca2+ext /Ca2+int | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Allicin | rat pulmonary | 0.8 µg/mL 1 | d | + | x | [65] | ||||
| 2 | Allyl isothiocyanate | rat cerebral | 164 µM 2 | d | x | x | +IKCa, | +TRPA1/ | [66] | ||
| +SKCa, | |||||||||||
| +KIR | |||||||||||
| 3 | Alpha-terpineol | rat mesenteric | NR | + | [67] | ||||||
| 4 | Alpha-zearalanol | rat aorta | NR | d/i | + | +BKCa, | -VOCC/ | [68] | |||
| +KATP | |||||||||||
| 5 | Alpinetin | rat mesenteric | 27.5 µM 1 | d/i | + | x | - | -VOCC/ -IP3R, -RyRs | [69] | ||
| 6 | Alstonisine | rat aorta | NR | d/i | + | x | -VOCC,- | [70] | |||
| ROCC/ | |||||||||||
| 7 | Amentoflavone | rat aorta | NR | d | + | x | + | -VOCC/ | [71] | ||
| 8 | Angelic ester of 2-β-hydroxy-8α-H-7(11)-eremophilene-12,8-olide | rat mesenteric | 4.74 ± 0.1 §,2 | -VOCCL/ | [72] | ||||||
| rat aorta | 5.43 ± 0.06 §,2 | ||||||||||
| 9 | Angelic ester of 2-β-hydroxy-8β-H-7(11)-eremophilene-12,8-olide | rat mesenteric | 4.11 ± 0.02 §,2 | x | x | -VOCCL/x | [72] | ||||
| rat aorta | 4.92 ± 0.09 §,2 | ||||||||||
| 10 | Apigenin | rat aorta | 3.7 ± 0.5 µM 1 | d/i | + x | x | x | +IKCa, +SKCa | -VOCC, -ROCC/x +TRPV4/ | [73] | |
| rat aorta | 63 µM 5 | i | [74] | ||||||||
| rat mesenteric | NR | d | [75] | ||||||||
| 11 | Apocynin | rat aorta | 780 ± 80 µM 1 | d/i | + | x | +KATP | -VOCC,- ROCC/ -IP3R | [76] | ||
| 12 | Astragaloside IV | rat aorta | NR | d/i | + | + | -VOCC,- ROCC/ -IP3R | [77] | |||
| 13 | Backebergine | rat aorta | NR | d/i | + | x | -VOCC,- ROCC/ | [78] | |||
| 14 | Baicalin | rat mesenteric | NR | i | + | + | - | +BKCa | -VOCC/ | [79] | |
| 15 | 4-Benzoyl-2-C-β-gluco-pyranosyl-3,5-dihydroxy-6-methylphenyl β-d-glucopyranoside | rat aorta | NR | d | + | [55] | |||||
| 16 | Berberine | rat mesenteric | 1.48 ± 0.16 µM 1 | d/i | + | x | x | +BKCa, +Kv, | x/-RyRs | [80] | |
| +KIR | |||||||||||
| 17 | Betulinic acid | rat aorta | 1.67 µM 1 | d | + | x | [81] | ||||
| 18 | Bilobalide | rat aorta | NR | + | x | -VOCC/ | [82] | ||||
| 19 | Biochanin A | rat aorta | NR | i | +BKCa, +KATP | -VOCC,- ROCC/- | [83] | ||||
| 20 | Brazilin | rat aorta | 183 ± 30 µM 1 | d | + | [84] | |||||
| rat aorta | i | x | [85] | ||||||||
| rat mesenteric | i | x | [85] | ||||||||
| 21 | (−)-Borneol | rat aorta | 4.63 ± 0.15 §,1 | i | +BKCa, +Kv, | -VOCCL/- | [86] | ||||
| +KATP | |||||||||||
| 22 | Butein | rat aorta | 7.4 ± 1.6 µM 1 | d | + | x | - | x | [87] | ||
| 23 | Butylidenephthalide | rat aorta | 4.20 ± 0.07 §,3 | d/i | + | x | - | x | -VOCCL, -ROCC/- | [88] | |
| 24 | Cadamine | rat aorta | NR | d/i | + | x | -VOCC,- ROCC/ | [89] | |||
| 25 | Caffeic acid | rat aorta | 400 µM 1 | d/i | + | x | [90] | ||||
| 26 | Caffeic acid phenethyl ester | porcine coronary | 4.99 ± 0.17 §,1 | d/i | + | -VOCC/ | [91] | ||||
| rat aorta | 5.15 ± 0.0 §,4 | d | + | x | [92] | ||||||
| 27 | Calycosin | rat aorta | 4.46 ± 0.13 §,3 | i | x | x | -VOCC/x | [93] | |||
| 28 | Capsaicin | rat mesenteric | NR | x | [94] | ||||||
| 29 | Cardamonin | rat mesenteric rat tail | 9.3 µM 1 4.63 ± 0.01 §,1 | d/i | + | x | - | +BKCa | -VOCC/- IP3R,-RyRs -VOCC/ | [69] [95] | |
| 30 | Carvacrol | rat aorta rat cerebral | 145.4 ± 6.07 µM 1 | id | x | x | - | +SKCa, +KIR, +IKCa | -VOCC/-IP3R +TRPV3/ | [96] [97] | |
| 78.8 ± 11.9 µM 2 | |||||||||||
| 4.1 µM | |||||||||||
| 31 | Cassiarin A | rat mesenteric | 6.4 ± 0.8 µM 1 | d/i | + | x | +BKCa | [98] | |||
| 32 | Cathafoline | rat aorta | NR | d/i | + | x | -ROCC/ | [70] | |||
| 33 | Centaureidin | rat orta | 16.7 ± 1.9 µM 5 | i | [99] | ||||||
| 34 | Chrysin | rat orta | 16 ± 4 µM 1 | d | + | [100,101] | |||||
| 35 | Chrysin glucoside | rat aorta | 52 µM 5 | d/i | + | [102] | |||||
| 36 | Cinnamaldehyde | rat aorta | NR | d/i | + | x | x | -VOCC/ | [103] | ||
| 37 | Ethyl cinnamate | rat aorta | 380 ± 40 µM 1 | d/i | + | + | -VOCC/ | [104] | |||
| 38 | 1,8-Cineole | rat aorta | 663.2 ± 63.8 µg/mL 1 | d | + | x | x | [105] | |||
| 39 | (+)-cis-4'-O-Acetyl-3'- O-angeloylkhellactone | rat aorta | NR | d/i | + | x | x | -VOCC/ | [106] | ||
| 40 | Citral | rat aorta | NR | d/i | + | x | -ROCC/- | [102] | |||
| 41 | Citronellol | rat mesenteric | 0.71 ± 0.11 §,1 | i | x | -VOCC/- IP3R, -RyRs | [107] | ||||
| 42 | Coptisine | rat aorta | 4.49 ± 0.48 §,5 | d/i | + | + | +KV | -VOCC,- ROCC/- | [108] | ||
| 43 | Cornuside | rat aorta | NR | d | + | x | x | [109] | |||
| 44 | Cryptotanshinone | rat coronary | 2.65 ± 0.15 µg/mL 6 | i | x | x | x | -VOCCL/ | [110] | ||
| 45 | Curine | rat mesentericrat aorta | 4.8 ± 1.9 µM 5 7.6 ± 1.6 µM 1 | i | -VOCC/- - VOCCL/- | [111] [112] | |||||
| 46 | Curcumin | porcine coronary | 6.28 ± 0.28 µM 4 | d | + | x | [113] | ||||
| 47 | Cyclosquamosin B | rat aorta | NR | i | -VOCC/ | [114] | |||||
| 48 | Daidzein | rat basilar | 20 ± 7 µM 3 7.4 ± 1.9 µM6 | ii | x | x | + +BKCa, +KATP | -VOCC/ | [115] [116] | ||
| 49 | Daidzin | rat basilar | 140 ± 21 µM 3 | i | x | x | +KATP | -VOCC/ | [115] | ||
| 50 | Danshensu | rat coronary | 71.5 ± 11 µg/mL 6 | i | + | -VOCCL/ | [117] | ||||
| 51 | Dehydroevodiamine | rat mesenteric | NR | d/i | + | x | + | -VOCC/ | [118] | ||
| 52 | Demethylpiperitol | rat aorta | NR | d | + | [119] | |||||
| 53 | Denudatin B | rat aorta | 21.2 µg/mL 2 | i | ↑cGMP | x | -VOCC,- ROCC/x | [120] | |||
| 54 | 14-Deoxy-andrographolide | rat aorta | NR | d /i | + | x | x | -VOCC, -ROCC/ | [121] | ||
| 55 | Dictamnine | rat aorta | 15 µM 2 | i | x | -VOCC,- ROCC/ | [122] | ||||
| 56 | Dihydrotanshinone | rat coronary | 10.39 ± 1.69 µM 6 | i | x | x | x | -VOCCL/ | [123] | ||
| 57 | 3,7-Dihydroxy-2,4-dimethoxyphenanthrene | rat aorta | NR | d/i | + | [124] | |||||
| 58 | Dioclein | rat aorta | 1.3 ± 3.1 µM 1 | d | + | x x | - | - | +KCa, +KV | -VOCC/-IP3R | [20] |
| rat aorta | 350 ± 80 µM 5 | i | [21] | ||||||||
| rat mesenteric | 0.3 ± 0.06 µM 1 | d/i | [22] | ||||||||
| human saphenous | 7.3 ± 3.1 µM 1 | i | [23] | ||||||||
| 59 | Diosgenin | rat mesenteric | 330 ± 120 µM 1 | d | + | + | +BKCa | [62] | |||
| 60 | Echinacoside | rat aorta | NR | d | + | x | [125] | ||||
| 61 | Ellagic acid | rat aorta | 5.60 ± 0.03 §,1 | d/i | + | x | x | -VOCCL/ | [126] | ||
| 62 | Emodin | rat aorta | NR | i | ↑cGMP | [127] | |||||
| 63 | Ent-18-hydroxy-trachyloban-3-one | rat aorta | 5.7 ± 0.01 §,2 | x | -VOCCL/ | [128] | |||||
| 64 | Ent-8(14), 15-pimaradien-3β-ol | rat aorta | 4.8 ± 0.1 §,1 | d/i | + | x | x | -VOCC/x | [129] | ||
| 65 | Epicatechin | rat aorta | 4.72 ± 0.07 §,1 | d | + | [130] | |||||
| 66 | 7-Epiclusianone | rat aorta | NR | d | + | x | [131] | ||||
| 67 | (−)-Epigallocatechin-3-gallate | rat aorta | 191.8 ± 13 µM 5 | i | - | x +BKCa | [29] | ||||
| bovine ophtalmic | 6.21 ± 0.06 §,6 | d | + | [31] | |||||||
| rat aorta | 4.76 ± 0.07 §,1 | d | + | [130] | |||||||
| 68 | Equol (daidzein metabolite) | rat aorta | NR | d | + | [132] | |||||
| 69 | Eriodictyol | rat aorta | 61.1 ± 2 µM 5 | i | x | -VOCC/ | [133] | ||||
| 70 | Erythrodiol | rat aorta | 3.38 ± 1.27 µM 1 | d | + | x | [134] | ||||
| 71 | Eudesmin | rat aorta | 10.69 ± 0.77 µg/mL 1 | d | + | + | [135] | ||||
| 72 | Eugenol | rat aorta | x | x | - VOCC,- ROCC/x - VOCC,- ROCC/ | [136] | |||||
| rat aorta | 1200 µM 1 | d/i | + | [137] | |||||||
| rat mesenteric | d/i | x | [138] | ||||||||
| 73 | Euxanthone | rat aorta | 32.5 ± 2.5 µM 5 | i | x | x | - | x | -VOCC,- ROCC/ -IP3R | [139] | |
| 74 | Evocarpine | rat aorta | 9.8 µM 2 | -VOCC/ | [140] | ||||||
| 75 | Evodiamine | rat mesenteric | NR | d/i | x | -ROCC/x | [141] | ||||
| 76 | Ferulic acid | rat aorta | NR | i | x | x/ | [142] | ||||
| 77 | Floranol | rat mesenteric | 19.9 ± 2.4 µM 1 | d/i | + | x | x | -VOCC/ | [143] | ||
| rat aorta | i | x | [144] | ||||||||
| 78 | Formononetin | rat aorta | NR | d/ i | + | + | -VOCC/ | [145] | |||
| 79 | Forsythide | rat aorta | NR | i | x | -ROCC/ | [146] | ||||
| 80 | Fraxinellone | rat aorta | 25 µM 2 | -VOCC/ | [122] | ||||||
| 81 | Galangin | rat aorta | NR | d/i | + | x | -VOCC/ | [147] | |||
| 82 | Geissoschizine methyl ether | rat aorta | 0.744 µM 5 | d/i | + | -VOCC/ | [148] | ||||
| 83 | Genistein | rabbit coronary | NR | i | x | x | x | -VOCCL/ | [149] | ||
| human umbilical | -VOCC/- | [150] | |||||||||
| 84 | Gigantol | rat aorta | NR | d/i | + | [124] | |||||
| 85 | Ginsenoside Rg3 | rat aorta | NR | d | + | + | [151] | ||||
| 86 | Gomisin A | rat aorta | NR | d/i | + | [152] | |||||
| 87 | Gymnopusin | rat aorta | 63 µM 5 | i | x | +BKCa, +KATP | -VOCCL/ | [153] | |||
| 88 | Harmaline | rat aorta | 32.8 ± 1.17 µM 2 | d/i | + | + | - | -VOCC/ | [154] | ||
| 89 | Harman | rat aorta | 9 µM 1 | d/i | + | x | x | -VOCCL, -ROCC/ | [155] | ||
| 90 | Harmine | rat aorta | 3.7 ± 1.2 µM 5 | i | x | x | - | -VOCC/ | [154] | ||
| 91 | Hematoxylin | rat aorta | NR | d | + | [156] | |||||
| 92 | Hesperetin | rat aorta | 62.8 ± 5.0 µM 5 | i | x | - | x | -VOCC,- ROCC/ | [157] | ||
| 93 | Hirsutine | rat aorta | 10.6 µM 5 | i | -VOCC/ | [148] | |||||
| 94 | 4-Hydroxybenzoic acid | rat aorta | 1780 µM 1 | d | + | x | [90] | ||||
| 95 | 4-Hydroxyderricin | rat aorta | NR | d/i | + | -VOCC/ | [53] | ||||
| 96 | 1-Hydroxy-2,3,5-trimethoxyxanthone | rat coronary | 1.67 ± 0.27 µM 6 | d | + | x | - | x | -VOCCL/x | [130] | |
| 97 | Hypogallic acid | rat aorta | 620 µM 1 | d/ i | + | +KATP | [90] | ||||
| 98 | Icariin | rat aorta | NR | + | [158] | ||||||
| canine coronary | d | + | x | x | [159] | ||||||
| 99 | Imperatorin | rat mesenteric mouse aorta | i | +BKCa | -VOCC,- ROCC/- | [160] | |||||
| 12.2 ± 2.4 µM 1 | d | + | [161] | ||||||||
| 100 | Isoliquiritigenin | rat aorta | 7.4 ± 1.6 µM 1 | i | ↑cGMP | x | x | [162] | |||
| 101 | Isoplagiochin B | rat aorta | NR | i | + | -ROCC/ | [56] | ||||
| 102 | Isoplagiochin D | rat aorta | NR | i | x | -VOCC,- ROCC/ | [56] | ||||
| 103 | Isopropyl 3-(3,4-dihydroxyphenyl) -2-hydroxypropanoate | rat mesenteric | 7.41 ± 0.08 §,5 | i | +BKCa | -VOCC,- ROCC/- | [123] | ||||
| 104 | Isorhamnetin | rat mesenteric | 5.89 ± 0.11 §,5 | i | x | x | [163] | ||||
| 105 | Isorhynchophylline | rat aorta | 20–30 µM 2 | i | x | -VOCCL/- IP3R | [164] | ||||
| 106 | Iso-S-petasin | rat aorta | NR | i | -VOCCL/ | [165] | |||||
| 107 | Isotirumalin | rat aorta | 4.84 ± 0.24 ǂ,1 | d | + | [166] | |||||
| 108 | Jatrophone | rat aorta | 11.0 µM 5 | d/i | + | -VOCC/- | [167] | ||||
| rat portal vein | 13.54 µM 5 | - | [168] | ||||||||
| 109 | Kaempferol | rat aorta rat aorta rat mesenteric porcine coronary rat aorta | 580 µM 1 4.81 ± 0.13 §,5 5.66 ± 0.06 §,5 | d/i d/i d | + + | [90] [163] [163] [169] [170] | |||||
| 110 | Kaurenoic acid | rat aorta | NR | d/i | + | x | +BKCa, +KV | -VOCC/x | [171] | ||
| 111 | Keayanidine B | rat aorta | 23.3 ± 1.3 µM 1 | + | [172] | ||||||
| 112 | Keayanine | rat aorta | 27.5 ± 2.4 µM 1 | + | [172] | ||||||
| 113 | Kolaviron | rat mesenteric | NR | i | +BKCa, +KV | -VOCCL/ - IP3R | [173] | ||||
| 114 | Labdane-302 | rat mesenteric | 5.4 ± 1.4 µM 1 | d/i | + | + | -VOCCL/ | [174] | |||
| 115 | Labd-8 (17)-en-15-oic acid | rat aorta | 313.6 µg/mL 2 | i | x | [175] | |||||
| 116 | Lectin (of Pisum arvense) | rat aorta | 58.38 ± 1.87 µg/mL 1 | d | + | x | x | [176] | |||
| 117 | Leonurine | rat aorta | 86.4 ± 10.4 µM 1 | - VOCCL/- | [177] | ||||||
| 118 | Leucocyanidol | rat aorta | 2.75 ± 0.15 §,5 | d/i | + | [178] | |||||
| 119 | Ligustilide | rat mesenteric | 3.98 §,2 | i | x | x | -VOCC,- ROCC/ -RyR | [179] | |||
| rat aorta | 4.39 ± 0.11 §,1 | i | x | x | x | [180] | |||||
| 120 | (−)-limacine | rat aorta | NR | d | + | [78] | |||||
| 121 | Luteolin | rat aorta | NR | i | x | +KIR, +KV | -VOCC/- | [17,181] | |||
| 122 | Machilin D | rat aorta | 17.8 µM | d | + | [182] | |||||
| 123 | Marrubenol | rat aorta | 11.8 ± 0.3 µM 2 | -VOCCL/ | [183] | ||||||
| 124 | Marrubiin | rat aorta | NR | d/i | + | -VOCC/ | [184] | ||||
| 125 | 10-Methoxyaffinisine | rat aorta | NR | d/i | + | x | -VOCC/ | [70] | |||
| 126 | Methyl brevifolincarboxylate | rat aorta | NR | i | -ROCC/x | [185] | |||||
| 127 | Methyleugenol | rat mesenteric | NR | d/i | + | [67] | |||||
| 128 | Methylpaeoniflorin | rat aorta | 10.1 µM 1 | d | + | [186] | |||||
| 129 | Milonine | rat mesenteric | 1.1 µM 1 | d/i | + | x | +BKCa, +SKCa, +KATP | - VOCC,- ROCC/ -IP3R,-RyR | [187] | ||
| 130 | Mollic acid glucoside | rat aorta | NR | d | + | [188] | |||||
| 131 | Morolic acid | rat aorta | 94.19 µM 5 | d | + | x | [189] | ||||
| 132 | Moronic acid | rat aorta | 16.11 µM 5 | d | + | x | [189] | ||||
| 133 | (+)-Nantenine | rat aorta | NR | i | x | -VOCC/x | [190] | ||||
| 134 | (+/−)-Naringenin | rat aortarat aortarat aorta | 71.2 ± 5.3 µM 1 4.68 µM 5 | i i i | - | - | +BKCa | -VDCC, -ROCC/ | [17] [18] [19] | ||
| 135 | Naucline | rat aorta | 20 µM 1 | i | x | -VOCC, -ROCC/ | [89] | ||||
| 136 | 1-Nitro-2-phenylethane | rat aorta | 231.5 µM 1 | i | + | x | +KATP, +KV | [191] | |||
| 137 | Norathyriol | rat aorta | NR | i | x | x | -VOCC, -ROCC/ | [192] | |||
| 138 | Oleanolic acid | rat aorta | 5.58 ± 1.28 µM 1 | d | + | x | [134] | ||||
| 139 | 12-O-Methylcurine | rat aorta | 63.2 ± 8.8 µM 1 | i | - | -VOCC,- ROCC/ -IP3R | [193] | ||||
| 140 | Orientin | New Zealand rabbit aorta | 2.28 µM 1 | d/i | + | x | x | - VOCC,- ROCC/- | [194] | ||
| 141 | Osthole | rat aorta | NR | i | ↑cGMP | x | - VOCC,- ROCC/- | [195] | |||
| 142 | Paeoniflorin | rat aorta | 19.4 µM 1 | d | + | [186] | |||||
| 143 | Paeonidanin | rat aorta | 7.9 µM 1 | d | + | [186] | |||||
| 144 | Pecrassipine A | rat aorta | NR | d/i | + | x | - VOCC,- ROCC/ | [78] | |||
| 145 | 1,2,3,4,6-Penta-O-galloyl-β-d-glucose | rat aorta | 3.6 µM 1 | d | + | + | x | [196] | |||
| 146 | Perrottetin | rat aorta | NR | i | x | - VOCC,- ROCC/ | [56] | ||||
| 147 | Phlomeoic acid | rat aorta | NR | d/i | + | -VOCC/ | [184] | ||||
| 148 | Phloretin | rabbit coronary | NR | i | [149] | ||||||
| 149 | Piceatannol | rat aorta | 2.4 ± 0.4 µM 1 | d | + | x | +BKCa | [32] | |||
| rat aorta | d | + | [33] | ||||||||
| 150 | Pimaradienoic acid | rat aorta | NR | i | + | + | x | -VOCC/x | [197] | ||
| 151 | Pinocembrin | rat aorta | 4.37 ± 0.02 §,5 | d/i | + | x | +KATP, +KV | - VOCC/- IP3R | [198] | ||
| 152 | Piperitol (sesamin metabolite) | rat aorta | NR | d | + | [119] | |||||
| 153 | Plagiochin A | rat aorta | NR | d | + | [56] | |||||
| 154 | Polygodial | rabbit pulmonary | NR | d | + | x | x | [37] | |||
| rat portal | - | -VOCC/ | [38] | ||||||||
| 155 | Pomolic acid | rat aorta | 2.45 μM 5 | d | + | x | +KATP | [199] | |||
| 156 | (+) Praeruptorin A | rat aorta | 35.4 ± 3.6 µM 1 | d | + | x | - VOCC,- ROCC/ -IP3R | [200] | |||
| 157 | (−) Praeruptorin A | rat aorta | 45.8 ± 2.5 µM 1 | i | x | x | -VOCC, -ROCC/ -IP3R | [200] | |||
| 158 | Proanthocyanidins* | rat aorta | NR | d | + | [50] | |||||
| 159 | Procyanidins* | human internal mammary | NR | d | + | + | +KATP, +SKCa, +KV, +KIR | [201] | |||
| rat aorta | d | + | [202] | ||||||||
| porcine coronary | + | + | [203] | ||||||||
| 160 | Protosappanin D | rat aortarat mesenteric | NR | d/i | + | + | [85] | ||||
| 161 | Puerarin | rat basilar | 304 ± 49 µM 3 | d/i | + | x | + | x/ | [115] | ||
| 162 | Quercetin | rat aorta | NR | i | x + + x | + x | x | - | +BKCa | [24] | |
| rat coronary | 3 mM 7 | d/i | [25] | ||||||||
| pig coronary | NR | i | [27] | ||||||||
| rat aorta | 4.68 ± 0.08 §,5 | i | [163] | ||||||||
| rat mesenteric | 5.35 ± 0.15 §,5 | i | [163] | ||||||||
| rat aorta | 4.36 ± 0.05 §,1 | d | [204] | ||||||||
| rat portal | 59.5 ± 11.1 µM 4 | i | [205] | ||||||||
| 163 | Quercetin 3,7-dimethyl ether | rat aorta | 4.70 ± 0.18 §,1 | d | + | [206] | |||||
| 164 | Quercetine-3-O-galactoside | rat basilar | 20.4 ± 4.49 µM 3 | d/i | + | + | + | [207] | |||
| 165 | Resveratrol | rat aorta | 4.52 ± 0.11 §,1 4.99 ± 0.11 §,1 | i | + | +KV +KV | -VOCC/ | [35] | |||
| rat aorta | d/i | [208] | |||||||||
| rat mesenteric | d/i | [209] | |||||||||
| 166 | Reticuline | rat aortarat aorta | 40 ± 10 µM 1 NR | d/i | + | x | - VOCCL/- IP3R -VOCCL/ | [63] [210] | |||
| 167 | Rhynchophylline | rat aorta | 20–30 µM 2 | i | x | - VOCCL/- IP3R,- RyR | [164] | ||||
| 168 | Riccardin A | rat aorta | NR | d | + | [56] | |||||
| 169 | Riccardin C | rat aorta | NR | d | + | [56] | |||||
| 170 | Riccardin F | rat aorta | NR | d | + | [56] | |||||
| 171 | Roseoside | rat aorta | NR | d | + | [55] | |||||
| 172 | Rotundifolone | rat aorta | 184 ± 6 µg/mL 1 | d/i | + | + | +BKCa | - VOCCL/- IP3R -VOCCL/ | [40] | ||
| rat aorta | NR | i | [41] | ||||||||
| rat mesenteric | 4.0 ± 0.02 §,1 | d/i | [42] | ||||||||
| 173 | Rutaecarpine | rat aorta | NR | d | + | x | -/- - VOCCL/- IP3R | [43] | |||
| rat aorta | d | + | [44] | ||||||||
| rat aorta | [45] | ||||||||||
| 174 | Rutin | rat mesenteric rat aorta | NR | d | + | + | +KATP | [211] | |||
| 175 | Salvianolic acid B | rat coronary | 147.9 ± 17.4 µg/mL 6 | i | + | -VOCC/ | [212] | ||||
| 176 | Sanguinarine | rat aorta | 3.18 ± 0.37 µM 1 | i | -VOCC, -ROCC/ -IP3R | [213] | |||||
| 177 | Saponins from Ginseng* | NR | -ROCC/ | [49] | |||||||
| 178 | Sappanchalcone | rat aortarat mesenteric | NR | d | + | + | [85] | ||||
| 179 | Saucerneol | rat aorta | 2.2 µM | d | + | [182] | |||||
| 180 | Saucerneol D | rat aorta | 12.7 µM | d | + | [182] | |||||
| 181 | Scirpusin B | rat aorta | NR | d | + | [214] | |||||
| 182 | Scutellarin | rat aorta | 7.7 ± 0.6 µM 5 | i | x | x | x | x | -VOCC/x | [215] | |
| 183 | Senkyunolide A | rat aorta | 4.32 ± 0.10 §,1 | i | x | x | x | [180] | |||
| 184 | S-petasin | rat mesenteric | 6.01 ± 0.08 §,3 | i i | x x | x x | - VOCCL/ - VOCCL/ | [72] | |||
| rat aorta | 4.76 ± 0.16 §,3 | [72] | |||||||||
| rat aorta | 6.6 ± 1.4 µM 2 | [216] | |||||||||
| 185 | Tetramethylpyrazine | rat aorta | NR | d/i | + + | +KATP, +SKCa | -VOCC/ | [217] | |||
| rabbit basilar | NR | [218] | |||||||||
| rat aorta | NR | [219] | |||||||||
| rat pulmonary | 522 µM 1 | [220] | |||||||||
| 186 | Tetrandrine | NR | -VOCCL/ | [217] | |||||||
| 187 | Thaligrisine | rat aorta | 23.0 ± 0.39 µM 5 | -VOCC/ | [221] | ||||||
| 188 | Thymol | rat aorta | 106.4 ± 11.3 µM 1 | i | x | - | -VOCC/-IP3R | [96] | |||
| 189 | Tilianin | rat aorta | 240 µM 5 | d/i | + | x | + KV | [222] | |||
| 190 | Trans-dehydrocrotonin | rat aorta | NR | d | + | [223] | |||||
| 191 | Trans-resveratrol | rat aorta | 3.12 ± 0.26 µM 1 | d | + | [224,225] | |||||
| 192 | Ursolic acid | rat aorta | 44.1 ± 6.1 µM 5 | d | + | x | [64] | ||||
| 193 | Villocarine A | rat aorta | NR | d/i | + | + | -VOCC, -ROCC/ | [226] | |||
| 194 | Vincamedine | rat aorta | NR | d/i | + | x | -VOCC, -ROCC/ | [227] | |||
| 195 | Visnadine | rat aortarat portal | NR | - | -VOCCL/ | [228] | |||||
| 196 | Visnagin | rat aorta | 22 ± 4 µM 5 | i | - | -VOCCL, -ROCC/ | [229] | ||||
| -IP3R,-RyR | |||||||||||
| 197 | Vitisin C | rabbit aorta | NR | d | + | [230] | |||||
| 198 | Vulgarenol | guinea pig heart | NR | d | + | [231] | |||||
| 199 | Wine polyphenolic compounds * | rat aorta | 3.27 ± 0.02 §,5 | d | + | x | + | [47,178] | |||
| 200 | Xanthoangelol | rat aorta | NR | d | + | -VOCC/ | [53] | ||||
| 201 | Xanthoangelol B | rat aorta | NR | i | x | -VOCC/ | [53] | ||||
| 202 | Xanthoangelol E | rat aorta | NR | d | + | -VOCC/ | [53] | ||||
| 203 | Xanthoangelol F | rat aorta | NR | d | + | -VOCC/ | [53] | ||||
| 204 | Xanthone | rat aorta | 60.26 ± 8.43 µM 5 | i | ↑cAMP | -VOCC, -ROCC/x | [232] | ||||
| 205 | Xanthorrhizol | rat aorta | NR | i | x | x | -VOCC, -ROCC/ | [233] | |||
| 206 | Zearalanone | rabbit coronary | NR | i | -VOCC/ | [149] | |||||
| 207 | (Z)-3-methylthioacrylic ester of 2beta-hydroxy-8betaH-7(11)-eremophilene-12,8-olide | rat mesentericrat aorta | 5.24 ± 0.13 §,3 4.26 ± 0.17 §,3 | i | x | x | -VOCCL/ | [72] |
6. Compounds Acting on the NO/cGMP Pathway
6.1. Compounds that Regulate eNOS Expression
6.2. Compounds that Regulate eNOS Activity
6.3. Compounds that Regulate the Activity and Expression of sGC
7. Compounds that Activate the PGI2/cAMP Pathway
8. Compounds that Inhibit Phosphodiesterases (PDEs)
9. Compounds that Activate K+ Channels
10. Compounds that Decrease Intracellular Ca2+ Concentration
11. Compounds that Activate Endothelial Transient Receptor Potential (TRP) Cation Channels
12. Compounds that Inhibit Protein Kinase C
13. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nguelefack, T.B.; Dongmo, A.B.; Dimo, T.; Kamanyi, A. Phytopharmacology of Some Medicinal Plants Used in Cameroonian Traditional Medicine to Handle Cardiovascular Diseases. In Recent Developments in Medicinal Plants Research; Capasso, A., Ed.; Research Signpost Publisher: Kerala, India, 2007; ISBN 978-81-308-0160-5. [Google Scholar]
- Cogolludo, A.; Pérez-Vizcaíno, F.; Tamargo, J. New insights in the pharmacological therapy of arterial hypertension. Curr. Opin. Nephrol. Hypertens. 2005, 14, 423–427. [Google Scholar]
- Brunner, H.; Cockcroft, J.R.; Deanfield, J. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 233–246. [Google Scholar] [CrossRef]
- Dominiczak, A.F.; Bohr, D.F. Nitric oxide and its putative role in hypertension. Hypertension 1995, 25, 1202–1211. [Google Scholar]
- Miles, A.M.; Bohle, D.S.; Glassbrenner, P.A.; Hansert, B.; Wink, D.A.; Grisham, M.B. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J. Biol. Chem. 1996, 271, 40–47. [Google Scholar]
- Bauersachs, J.; Bouloumie, A.; Fraccarollo, D.; Busse, R.; Ertl, G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 1999, 100, 292–298. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.C.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [Green Version]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharmacol. 2009, 157, 527–536. [Google Scholar] [CrossRef]
- Kloss, S.; Bouloumie, A.; Mulsch, A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension 2000, 35, 43–47. [Google Scholar] [CrossRef]
- Tsai, E.J.; Kass, D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009, 122, 216–238. [Google Scholar] [CrossRef]
- Crawley, D.E.; Zhao, L.; Giembycz, M.A.; Liu, S.; Barnes, P.J.; Winter, R.J.; Evans, T.W. Chronic hypoxia impairs soluble guanylyl cyclase-mediated pulmonary arterial relaxation in the rat. Am. J. Physiol. 1992, 263, L325–L332. [Google Scholar]
- Tzao, C.; Nickerson, P.A.; Russell, J.A.; Gugino, S.F.; Steinhorn, R.H. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr. Pulmonol. 2001, 31, 97–105. [Google Scholar] [CrossRef]
- Marijic, J.; Li, Q.; Song, M.; Nishimaru, K.; Stefani, E.; Toro, L. Decreased expression of voltage- and Ca (2+)-activated K(+) channels in coronary smooth muscle during aging. Circ. Res. 2001, 88, 210–216. [Google Scholar] [CrossRef]
- Hibino, H.; Kurachi, Y. A new insight into the pathogenesis of coronary vasospasm. Circ. Res. 2006, 98, 579–581. [Google Scholar] [CrossRef]
- Chan, E.; Woodman, O. Enhanced role for the opening of potassium channels in relaxant responses to acetylcholine after myocardial ischaemia and reperfusion in dog coronary arteries. Br. J. Pharmacol. 1999, 126, 925–932. [Google Scholar] [CrossRef]
- Jepps, T.A.; Chadha, P.S.; Davis, A.J.; Harhun, M.I.; Cockerill, G.W.; Olesen, S.P.; Hansen, R.S.; Greenwood, I.A. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 2011, 124, 602–611. [Google Scholar] [CrossRef]
- Sánchez de Rojas, V.R.; Somoza, B.; Ortega, T.; Villar, A.M. Different mechanisms involved in the vasorelaxant effect of flavonoids isolated from Satureja obovata. Planta Med. 1996, 62, 554–556. [Google Scholar] [CrossRef]
- Orallo, F.; Camiña, M.; Alvarez, E.; Basaran, H.; Lugnier, C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (+/−)-naringenin. Planta Med. 2005, 71, 99–107. [Google Scholar] [CrossRef]
- Saponara, S.; Testai, L.; Iozzi, D.; Martinotti, E.; Martelli, A.; Chericoni, S.; Sgaragli, G.; Fusi, F.; Calderone, V. (+/−)-Naringenin as large conductance Ca(2+)-activated K+ (BKCa) channel opener in vascular. Br. J. Pharmacol. 2006, 149, 1013–1021. [Google Scholar]
- Lemos, V.S.; Freitas, M.R.; Muller, B.; Lino, Y.D.; Queiroga, C.E.; Côrtes, S.F. Dioclein, a new nitric oxide- and endothelium-dependent vasodilator flavonoid. Eur. J. Pharmacol. 1999, 386, 41–46. [Google Scholar] [CrossRef]
- Trigueiro, F.; Cortes, S.F.; Almeida, R.N.; Lemos, V.S. Endothelium-independent vasorelaxant effect of dioclein, a new flavonoid isolated from Dioclea grandiflora, in the rat aorta. J. Pharm. Pharmacol. 2000, 52, 1431–1434. [Google Scholar]
- Côrtes, S.F.; Rezende, B.A.; Corriu, C.; Medeiros, I.A.; Teixeira, M.M.; Lopes, M.J.; Lemos, V.S. Pharmacological evidence for the activation of potassium channels as the mechanism involved in the hypotensive and vasorelaxant effect of dioclein in rat small resistance arteries. Br. J. Pharmacol. 2001, 133, 849–858. [Google Scholar] [CrossRef]
- Gonçalves, R.L.; Lugnier, C.; Keravis, T.; Lopes, M.J.; Fantini, F.A.; Schmitt, M.; Cortes, S.F.; Lemos, V.S. The flavonoid dioclein is a selective inhibitor of cyclic nucleotide phosphodiesterase type 1 (PDE1) and a cGMP-dependent protein kinase (PKG) vasorelaxant in human vascular tissue. Eur. J. Pharmacol. 2009, 620, 78–83. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Vizcaíno, F.; Zarzuelo, A.; Jiménez, J.; Tamargo, J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1993, 239, 1–7. [Google Scholar] [CrossRef]
- Kubota, Y.; Tanaka, N.; Umegaki, K.; Takenaka, H.; Mizuno, H.; Nakamura, K.; Shinozuka, K.; Kunitomo, M. Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001, 69, 2327–2336. [Google Scholar] [CrossRef]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O'Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 2008, 202, 58–67. [Google Scholar]
- Suri, S.; Liu, X.H.; Rayment, S.; Hughes, D.A.; Kroon, P.A.; Needs, P.W.; Taylor, M.A.; Tribolo, S.; Wilson, V.G. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br. J. Pharmacol. 2010, 159, 566–575. [Google Scholar] [CrossRef]
- Li, P.G.; Sun, L.; Han, X.; Ling, S.; Gan, W.T.; Xu, J.W. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology 2012, 89, 220–228. [Google Scholar] [CrossRef]
- Alvarez, E.; Campos-Toimil, M.; Justiniano-Basaran, H.; Lugnier, C.; Orallo, F. Study of the mechanisms involved in the vasorelaxation induced by (−)-epigallocatechin-3-gallate in rat aorta. Br. J. Pharmacol. 2006, 147, 269–280. [Google Scholar] [CrossRef]
- Campos-Toimil, M.; Orallo, F. Effects of (−)-epigallocatechin-3-gallate in Ca2+-permeable non-selective cation channels and voltage-operated Ca2+ channels in vascular smooth muscle cells. Life Sci. 2007, 80, 2147–2153. [Google Scholar] [CrossRef]
- Romano, M.R.; Lograno, M.D. Epigallocatechin-3-gallate relaxes the isolated bovine ophthalmic artery: Involvement of phosphoinositide 3-kinase-Akt-nitric oxide/cGMP signalling pathway. Eur. J. Pharmacol. 2009, 608, 48–53. [Google Scholar] [CrossRef]
- Oh, K.S.; Ryu, S.Y.; Kim, Y.S.; Lee, B.H. Large conductance Ca2+-activated K+ (BKCa) channels are involved in the vascular relaxations elicited by piceatannol isolated from Rheum undulatum rhizome. Planta Med. 2007, 73, 1441–1446. [Google Scholar] [CrossRef]
- Yoo, M.Y.; Oh, K.S.; Lee, J.W.; Seo, H.W.; Yon, G.H.; Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Lee, B.H. Vasorelaxant effect of stilbenes from rhizome extract of rhubarb (Rheum undulatum) on the contractility of rat aorta. Phytother. Res. 2007, 21, 186–189. [Google Scholar] [CrossRef]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Novakovic, A.; Bukarica, L.G.; Kanjuh, V.; Heinle, H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin. Pharmacol. Toxicol. 2006, 99, 360–364. [Google Scholar] [CrossRef]
- Wang, N.; Ko, S.H.; Chai, W.; Li, G.; Barrett, E.J.; Tao, L.; Cao, W.; Liu, Z. Resveratrol recruits rat muscle microvasculature via a nitric oxide-dependent mechanism that is blocked by TNFα. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E195–E201. [Google Scholar] [CrossRef]
- André, E.; Malheiros, A.; Cechinel-Filho, V.; Yunes, R.A.; Calixto, J.B. Mechanisms underlying the relaxation caused by the sesquiterpene polygodial in vessels from rabbit and guinea-pig. Eur. J. Pharmacol. 1999, 386, 47–53. [Google Scholar] [CrossRef]
- El Sayah, M.; Filho, V.C.; Yunes, R.A.; Malheiros, A.; Calixto, J.B. Action of polygodial on agonist-induced contractions of the rat portal vein in vitro. J. Cardiovasc. Pharmacol. 2000, 35, 670–675. [Google Scholar] [CrossRef]
- André, E.; Malheiros, A.; Cechinel-Filho, V.; Yunes, R.A.; Calixto, J.B. Role of nitric oxide and K+ channels in relaxation induced by polygodial in rabbit corpus cavernosum in vitro. J. Cardiovasc. Pharmacol. 2003, 41, 300–306. [Google Scholar] [CrossRef]
- Guedes, D.N.; Silva, D.F.; Barbosa-Filho, J.M.; Medeiros, I.A. Muscarinic agonist properties involved in the hypotensive and vasorelaxant responses of rotundifolone in rats. Planta Med. 2002, 68, 700–4. [Google Scholar] [CrossRef]
- Guedes, D.N.; Silva, D.F.; Barbosa-Filho, J.M.; Medeiros, I.A. Calcium antagonism and the vasorelaxation of the rat aorta induced by rotundifolone. Braz. J. Med. Biol. Res. 2004, 37, 1881–1887. [Google Scholar]
- Silva, D.F.; Araújo, I.G.; Albuquerque, J.G.; Porto, D.L.; Dias, K.L.; Cavalcante, K.V.; Veras, R.C.; Nunes, X.P.; Barbosa-Filho, J.M.; Araújo, D.A.; et al. Rotundifolone-induced relaxation is mediated by BK(Ca) channel activation and Ca(v) channel inactivation. Basic Clin. Pharmacol. Toxicol. 2011, 109, 465–475. [Google Scholar] [CrossRef]
- Chiou, W.F.; Chou, C.J.; Liao, J.F.; Sham, A.Y.; Chen, C.F. The mechanism of the vasodilator effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Eur. J. Pharmacol. 1994, 257, 59–66. [Google Scholar] [CrossRef]
- Chiou, W.F.; Liao, J.F.; Chen, C.F. Comparative study of the vasodilatory effects of three quinazoline alkaloids isolated from Evodia rutaecarpa. J. Nat. Prod. 1996, 59, 374–378. [Google Scholar] [CrossRef]
- Wang, G.J.; Wu, X.C.; Chen, C.F.; Lin, L.C.; Huang, Y.T.; Shan, J.; Pang, P.K. Vasorelaxing action of rutaecarpine: effects of rutaecarpine on calcium channel activities in vascular endothelial and smooth muscle cells. J. Pharmacol. Exp. Ther. 1999, 289, 1237–1244. [Google Scholar]
- Hu, C.P.; Xiao, L.; Deng, H.W.; Li, Y.J. The depressor and vasodilator effects of rutaecarpine are mediated by calcitonin gene-related peptide. Planta Med. 2003, 69, 125–129. [Google Scholar] [CrossRef]
- Andriambeloson, E.; Stoclet, J.C.; Andriantsitohaina, R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J. Cardiovasc. Pharmacol. 1999, 33, 248–254. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Dirsch, V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide 2009, 21, 77–91. [Google Scholar] [CrossRef]
- Kwan, C.Y. Vascular effects of selected antihypertensive drugs derived from traditional medicinal herbs. Clin. Exp. Pharmacol. Physiol. Suppl. 1995, 22, S297–S299. [Google Scholar] [CrossRef]
- Kawakami, K.; Aketa, S.; Sakai, H.; Watanabe, Y.; Nishida, H.; Hirayama, M. Antyhipertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2011, 75, 1435–1439. [Google Scholar] [CrossRef]
- Yung, L.M.; Leung, F.P.; Wong, W.T.; Tian, X.Y.; Yung, L.H.; Chen, Z.Y.; Yao, X.Q.; Huang, Y. Tea polyphenols benefit vascular function. Inflammopharmacology 2008, 16, 230–234. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.G.; Wang, M.Z.; Che, C.T.; Yeung, J.H. Vasodilatory actions of xanthones isolated from a Tibetan herb. Halenia elliptica. Phytomedicine 2009, 16, 1144–1150. [Google Scholar] [CrossRef]
- Matsuura, M.; Kimura, Y.; Nakata, K.; Baba, K.; Okuda, H. Artery relaxation by chalcones isolated from the roots of Angelica keiskei. Planta Med. 2001, 67, 230–235. [Google Scholar] [CrossRef]
- Shi, C.C.; Liao, J.F.; Chen, C.F. Comparative study on the vasorelaxant effects of three harmala alkaloids in vitro. Jpn. J. Pharmacol. 2001, 85, 299–305. [Google Scholar] [CrossRef]
- Lee, T.H.; Wang, G.J.; Lee, C.K.; Kuo, Y.H.; Chou, C.H. Inhibitory effects of glycosides from the leaves of Melaleuca quinquenervia on vascular contraction of rats. Planta Med. 2002, 68, 492–496. [Google Scholar] [CrossRef]
- Morita, H.; Zaima, K.; Koga, I.; Saito, A.; Tamamoto, H.; Okazaki, H.; Kaneda, T.; Hashimoto, T.; Asakawa, Y. Vasorelaxant effects of macrocyclic bis(bibenzyls) from liverworts. Bioorg. Med. Chem. 2011, 19, 4051–4056. [Google Scholar] [CrossRef]
- Triggle, C.R.; Samson, M.S.; Shalini, R.; Isra, M.; Gnanapragasam, A.; Hong, D. The endothelium: influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endohelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. (Greenwich) 2012, 14, 198–204. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Bunting, S.; Moncada, S.; Flower, R.J.; Vane, J.R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins 1976, 12, 685–713. [Google Scholar]
- Edwards, G.; Félétou, M.; Weston, A.H. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflugers Arch. 2010, 459, 863–879. [Google Scholar] [CrossRef]
- Dias, K.L.G.; Correia, N.A.; Pereira, K.K.G.; Barbosa-Filho, J.M.; Cavalcante, K.V.M.; Araújo, I.G.A.; Silva, D.F.; Guedes, D.N.; Neto, M.A.; Bendhack, L.M.; et al. Mechanisms involved in the vasodilator effect induced by diosgenin in rat superior mesenteric artery. Eur. J. Pharmacol. 2007, 574, 172–178. [Google Scholar] [CrossRef]
- Dias, K.L.; da Silva Dias, C.; Barbosa-Filho, J.M.; Almeida, R.N.; de Azevedo Correia, N.; Medeiros, I.A. Cardiovascular effects induced by reticuline in normotensive rats. Planta Med. 2004, 70, 328–333. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; López-Guerrero, J.; Navarrete-Vázquez, G.; Estrada-Soto, S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef]
- Ku, D.D.; Abdel-Razek, T.T.; Dai, J.; Kim-Park, S.; Fallon, M.B.; Abrams, G.A. Garlic and its active metabolite allicin produce endothelium- and nitric oxide-dependent relaxation in rat pulmonary arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 84–91. [Google Scholar] [CrossRef]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Lahlou, S.; Jucá, D.M.; Coelho-de-Souza, L.N.; da Frota, P.T.; da Costa, A.M.; Leal-Cardoso, J.H. Vasorelaxation induced by the essential oil of Croton nepetaefolius and its constituents in rat aorta are partially mediated by the endothelium. Fundam. Clin. Pharmacol. 2008, 22, 169–177. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, D.; Zhu, Y.; Liu, W.; Duan, J.; Dai, S. Relaxing effects of phytoestrogen alpha-zearalanol on rat thoracic aorta rings in vitro. Chin. J. Physiol. 2009, 52, 99–105. [Google Scholar] [CrossRef]
- Wang, Z.T.; Lau, C.W.; Chan, F.L.; Yao, X.; Chen, Z.Y.; He, Z.D. Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum. J. Cardiovasc. Pharmacol. 2001, 37, 596–606. [Google Scholar] [CrossRef]
- Zaima, K.; Koga, I.; Iwasawa, N.; Hosoya, T.; Hirasawa, Y.; Kaneda, T.; Ismail, I.S.; Lajis, N.H.; Morita, H. Vasorelaxant activity of indole alkaloids from Tabernaemontana dichotoma. J. Nat. Med. 2013, 67, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.G.; Yin, M.H.; Oh, H.; Lee, D.H.; Lee, H.S. Vasorelaxation by amentoflavone isolated from Selaginella tamariscina. Planta Med. 2004, 70, 718–722. [Google Scholar] [CrossRef]
- Sheykhzade, M.; Smajilovic, S.; Issa, A.; Haunso, S.; Christensen, S.B.; Tfelt-Hansen, J. S-262 and butterbur lactones dilate vessels through blockage of voltage gated calcium channels and block DNA synthesis. Eur. J. Pharmacol. 2008, 593, 79–86. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Park, Y.S.; Kim, T.J.; Fang, L.H.; Ahn, H.Y.; Hong, J.T.; Kim, Y.; Lee, C.K.; Yun, Y.P. Endothelium-dependent vasorelaxant and antiproliferative effects of apigenin. Gen. Pharmacol. 2000, 35, 341–347. [Google Scholar] [CrossRef]
- Ko, F.N.; Huang, T.F.; Teng, C.M. Vasodilatory action mechanisms of apigenin isolated from Apium graveolens in rat thoracic aorta. Biochim. Biophys. Acta 1991, 1115, 69–74. [Google Scholar]
- Ma, X.; He, D.; Ru, X.; Chen, Y.; Cai, Y.; Bruce, I.C.; Xia, Q.; Yao, X. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br. J. Pharmacol. 2012, 166, 349–358. [Google Scholar] [CrossRef]
- Senejoux, F.; Girard-Thernier, C.; Berthelot, A.; Bévalot, F.; Demougeot, C. New insights into the mechanisms of the vasorelaxant effects of apocynin in rat thoracic aorta. Fundam. Clin. Pharmacol 2012. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.H.; Zhong, M.F.; Liu, R.H.; Li, H.L.; Zhang, W.D.; Chen, H. Mechanisms underlying vasorelaxant action of astragaloside IV in isolated rat aortic rings. Clin. Exp. Pharmacol. Physiol. 2007, 34, 387–392. [Google Scholar] [CrossRef]
- Zaima, K.; Takeyama, Y.; Koga, I.; Saito, A.; Tamamoto, H.; Azziz, S.S.; Mukhtar, M.R.; Awang, K.; Hadi, A.H.; Morita, H. Vasorelaxant effect of isoquinoline derivatives from two species of Popowia perakensis and Phaeanthus crassipetalus on rat aortic artery. J. Nat. Med. 2012, 66, 421–427. [Google Scholar] [CrossRef]
- Lin, Y.L.; Dai, Z.K.; Lin, R.J.; Chu, K.S.; Chen, I.J.; Wu, J.R.; Wu, B.N. Baicalin, a flavonoid from Scutellaria baicalensis Georgi, activates large-conductance Ca2+-activated K+ channels via cyclic nucleotide-dependent protein kinases in mesenteric artery. Phytomedicine 2010, 17, 760–770. [Google Scholar] [CrossRef]
- Ko, W.H.; Yao, X.Q.; Lau, C.W.; Law, W.I.; Chen, Z.Y.; Kwok, W.; Ho, K.; Huang, Y. Vasorelaxant and antiproliferative effects of berberine. Eur. J. Pharmacol. 2000, 399, 187–196. [Google Scholar] [CrossRef]
- Fu, J.Y.; Qian, L.B.; Zhu, L.G.; Liang, H.T.; Tan, Y.N.; Lu, H.T.; Lu, J.F.; Wang, H.P.; Xia, Q. Betulinic acid ameliorates endothelium-dependent relaxation in L-NAME-induced hypertensive rats by reducing oxidative stress. Eur. J. Pharm. Sci. 2011, 44, 385–391. [Google Scholar] [CrossRef]
- Nishida, S.; Satoh, H. Mechanisms for the vasodilations induced by Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Life Sci. 2003, 72, 2659–2667. [Google Scholar] [CrossRef]
- Wang, H.P.; Mei, R.H.; Li, X.Y.; Zhao, M.H.; Lu, Y.; Xia, Q.; Bruce, I. Endothelium-independent vasorelaxant effect of the phyto-oestrogen biochanin a on rat thoracic aorta. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 3, 2244–2247. [Google Scholar]
- Hu, C.M.; Kang, J.J.; Lee, C.C.; Li, C.H.; Liao, J.W.; Cheng, Y.W. Induction of vasorelaxation through activation of nitric oxide synthase in endothelial cells by brazilin. Eur. J. Pharmacol. 2003, 468, 37–45. [Google Scholar] [CrossRef]
- Sasaki, Y.; Suzuki, M.; Matsumoto, T.; Hosokawa, T.; Kobayashi, T.; Kamata, K.; Nagumo, S. Vasorelaxant activity of Sappan lignum constituents and extracts on rat aorta and mesenteric artery. Biol. Pharm. Bull. 2010, 33, 1555–1560. [Google Scholar] [CrossRef]
- Silva-Filho, J.C.; Oliveira, N.N.; Arcanjo, D.D.; Quintans-Júnior, L.J.; Cavalcanti, S.C.; Santos, M.R.; Oliveira, R.D.; Oliveira, A.P. Investigation of mechanisms involved in (−)-Borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin. Pharmacol. Toxicol. 2011, 110, 171–177. [Google Scholar]
- Yu, S.M.; Cheng, Z.J.; Kuo, S.C. Endothelium-dependent relaxation of rat aorta by butein, a novel cyclic AMP-specific phosphodiesterase inhibitor. Eur. J. Pharmacol. 1995, 280, 69–77. [Google Scholar] [CrossRef]
- Chan, S.S.; Choi, A.O.; Jones, R.L.; Lin, G. Mechanisms underlying the vasorelaxing effects of butylidenephthalide, an active constituent of Ligusticum chuanxiong, in rat isolated aorta. Eur. J. Pharmacol. 2006, 537, 111–117. [Google Scholar] [CrossRef]
- Ishizuka, M.; Koga, I.; Zaima, K.; Kaneda, T.; Hirasawa, Y.; Hadi, A.H.; Morita, H. Vasorelaxant effects on rat aortic artery by two types of indole alkaloids, naucline and cadamine. J. Nat. Med. 2013, 67, 399–403. [Google Scholar] [CrossRef]
- Leeya, Y.; Mulvany, M.J.; Queiroz, E.F.; Marston, A.; Hostettmann, K.; Jansakul, C. Hypotensive activity of an n-butanol extract and their purified compounds from leaves of Phyllanthus acidus (L.) Skeels in rats. Eur. J. Pharmacol. 2010, 649, 301–313. [Google Scholar] [CrossRef]
- Cicala, C.; Morello, S.; Iorio, C.; Capasso, R.; Borrelli, F.; Mascolo, N. Vascular effects of caffeic acid phenethyl ester (CAPE) on isolated rat thoracic aorta. Life Sci. 2003, 73, 73–80. [Google Scholar] [CrossRef]
- Long, Y.; Han, M.; Chen, J.; Tian, X.Z.; Chen, Q.; Wang, R. The vasorelaxant effect of caffeic acid phenethyl ester on porcine coronary artery ring segments. Vascul. Pharmacol. 2009, 51, 78–83. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, Y.Y.; Cheng, J.; Zhao, Y.Y. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacol. Sin. 2006, 27, 1007–1012. [Google Scholar] [CrossRef]
- Potenza, M.A.; De Salvatore, G.; Montagnani, M.; Serio, M.; Mitolo-Chieppa, D. Vasodilatation induced by capsaicin in rat mesenteric vessels is probably independent of nitric oxide synthesis. Pharmacol. Res. 1994, 30, 253–261. [Google Scholar] [CrossRef]
- Fusi, F.; Cavalli, M.; Mulholland, D.; Crouch, N.; Coombes, P.; Dawson, G.; Bova, S.; Sgaragli, G.; Saponara, S. Cardamonin is a bifunctional vasodilator that inhibits Ca(v)1.2 current and stimulates K(Ca)1.1 current in rat tail artery myocytes. J. Pharmacol. Exp. Ther. 2010, 332, 531–540. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar]
- Earley, S.; Gonzales, A.L.; Garcia, Z.I. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol. Pharmacol. 2010, 77, 612–620. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Ishida, K.; Hirasawa, Y.; Morita, H.; Honda, T.; Kamata, K. Vasodilator effect of Cassiarin A, a novel antiplasmodial alkaloid from Cassia siamea, in rat isolated mesenteric artery. Biol. Pharm. Bull. 2010, 33, 844–848. [Google Scholar] [CrossRef]
- Orallo, F.; Lamela, M.; Camiña, M.; Uriarte, E.; Calleja, J.M. Preliminary study of the potential vasodilator effects on rat aorta of centaurein and centaureidin, two flavonoids from Centaurea corcubionensis. Planta Med. 1998, 64, 116–119. [Google Scholar] [CrossRef]
- Villar, I.C.; Vera, R.; Galisteo, M.; O’Valle, F.; Romero, M.; Zarzuelo, A.; Duarte, J. Endothelial nitric oxide production stimulated by the bioflavonoid chrysin in rat isolated aorta. Planta Med. 2005, 71, 829–834. [Google Scholar] [CrossRef]
- Duarte, J.; Jiménez, R.; Villar, I.C.; Pérez-Vizcaíno, F.; Jiménez, J.; Tamargo, J. Vasorelaxant effects of the bioflavonoid chrysin in isolated rat aorta. Planta Med. 2001, 67, 567–569. [Google Scholar] [CrossRef]
- Devi, R.C.; Sim, S.M.; Ismail, R. Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid. Based Complement. Alternat Med. 2012, 2012, 539475:1–539475:8. [Google Scholar]
- Yanaga, A.; Goto, H.; Nakagawa, T.; Hikiami, H.; Shibahara, N.; Shimada, Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol. Pharm. Bull. 2006, 29, 2415–2418. [Google Scholar] [CrossRef]
- Othman, R.; Ibrahim, H.; Mohd, M.A.; Awang, K.; Gilani, A.U.; Mustafa, M.R. Vasorelaxant effects of ethyl cinnamate isolated from Kaempferia galanga on smooth muscles of the rat aorta. Planta Med. 2002, 68, 655–657. [Google Scholar] [CrossRef]
- Pinto, N.V.; Assreuy, A.M.; Coelho-de-Souza, A.N.; Ceccatto, V.M.; Magalhães, P.J.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef]
- Lee, J.W.; Roh, T.C.; Rho, M.C.; Kim, Y.K.; Lee, H.S. Mechanisms of relaxant action of a pyranocoumarin from Peucedanum japonicum in isolated rat thoracic aorta. Planta Med. 2002, 68, 891–895. [Google Scholar] [CrossRef]
- Bastos, J.F.; Moreira, I.J.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; De Sousa, D.P.; Santos, M.R. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 331–337. [Google Scholar]
- Gong, L.L.; Fang, L.H.; Qin, H.L.; Lv, Y.; Du, G.H. Analysis of the mechanisms underlying the vasorelaxant action of coptisine in rat aortic rings. Am. J. Chin. Med. 2012, 40, 309–320. [Google Scholar] [CrossRef]
- Kang, D.G.; Choi, D.H.; Lee, J.K.; Lee, Y.J.; Moon, M.K.; Yang, S.N.; Kwon, T.O.; Kwon, J.W.; Kim, J.S.; Lee, H.S. Endothelial NO/cGMP-dependent vascular relaxation of cornuside isolated from the fruit of Cornus officinalis. Planta Med. 2007, 73, 1436–1440. [Google Scholar] [CrossRef]
- Lam, F.F.; Yeung, J.H.; Chan, K.M.; Or, P.M. Mechanisms of the dilator action of cryptotanshinone on rat coronary artery. Eur. J. Pharmacol. 2008, 578, 253–260. [Google Scholar] [CrossRef]
- Dias, C.S.; Barbosa-Filho, J.M.; Lemos, V.S.; Côrtes, S.F. Mechanisms involved in the vasodilator effect of curine in rat resistance arteries. Planta Med. 2002, 68, 1049–1051. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Pinho, J.F.; De-Lira, D.P.; Barbosa-Filho, J.M.; Araújo, D.A.; Cortes, S.F.; Lemos, V.S.; Cruz, J.S. Curine, a bisbenzylisoquinoline alkaloid, blocks L-type Ca2+ channels and decreases intracellular Ca2+ transients in A7r5 cells. Eur. J. Pharmacol. 2011, 669, 100–107. [Google Scholar] [CrossRef]
- Xu, P.H.; Long, Y.; Dai, F.; Liu, Z.L. The relaxant effect of curcumin on porcine coronary arterial ring segments. Vascul. Pharmacol. 2007, 47, 25–30. [Google Scholar] [CrossRef]
- Morita, H.; Iizuka, T.; Choo, C.Y.; Chan, K.L.; Takeya, K.; Kobayashi, J. Vasorelaxant activity of cyclic peptide, cyclosquamosin B, from Annona squamos. Bioorg. Med. Chem. Lett. 2006, 16, 4609–4611. [Google Scholar]
- Deng, Y.; Ng, E.S.; Yeung, J.H.; Kwan, Y.W.; Lau, C.B.; Koon, J.C.; Zhou, L.; Zuo, Z.; Leung, P.C.; Fung, K.P.; et al. Mechanisms of the cerebral vasodilator actions of isoflavonoids of Gegen on rat isolated basilar artery. J. Ethnopharmacol. 2012, 139, 294–304. [Google Scholar] [CrossRef]
- Zhang, H.T.; Wang, Y.; Deng, X.L.; Dong, M.Q.; Zhao, L.M.; Wang, Y.W. Daidzein relaxes rat cerebral basilar artery via activation of large-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Eur. J. Pharmacol. 2010, 630, 100–106. [Google Scholar] [CrossRef]
- Lam, F.F.; Yeung, J.H.; Chan, K.M.; Or, P.M. Relaxant effects of danshen aqueous extract and its constituent danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vascul. Pharmacol. 2007, 46, 271–277. [Google Scholar] [CrossRef]
- Chiou, W.F.; Liao, J.F.; Shum, A.Y.; Chen, C.F. Mechanisms of vasorelaxant effect of dehydroevodiamine: A bioactive isoquinazolinocarboline alkaloid of plant origin. J. Cardiovasc. Pharmacol. 1996, 27, 845–853. [Google Scholar] [CrossRef]
- Nakano, D.; Kwak, C.J.; Fujii, K.; Ikemura, K.; Satake, A.; Ohkita, M.; Takaoka, M.; Ono, Y.; Nakai, M.; Tomimori, N.; et al. Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: Possible involvement of the metabolites in the antihypertensive effect of sesamin. J. Pharmacol. Exp. Ther. 2006, 318, 328–335. [Google Scholar] [CrossRef]
- Yu, S.M.; Chen, C.C.; Huang, Y.L.; Tsai, C.W.; Lin, C.H.; Huang, T.F.; Teng, C.M. Vasorelaxing effect in rat thoracic aorta caused by denudatin B, isolated from the Chinese herb, magnolia fargesii. Eur. J. Pharmacol. 1990, 187, 39–47. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Tan, B.K. Vasorelaxation of rat thoracic aorta caused by 14-deoxyandrographolide. Clin. Exp. Pharmacol. Physiol. 1998, 25, 424–429. [Google Scholar] [CrossRef]
- Yu, S.M.; Ko, F.N.; Su, M.J.; Wu, T.S.; Wang, M.L.; Huang, T.F.; Teng, C.M. Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers. Naunyn Schmiedebergs Arch. Pharmacol. 1992, 345, 349–355. [Google Scholar]
- Wang, S.P.; Zang, W.J.; Kong, S.S.; Yu, X.J.; Sun, L.; Zhao, X.F.; Wang, S.X.; Zheng, X.H. Vasorelaxant effect of isopropyl 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate, a novel metabolite from Salvia miltiorrhiza, on isolated rat mesenteric artery. Eur. J. Pharmacol. 2008, 579, 283–288. [Google Scholar] [CrossRef]
- Estrada-Soto, S.; López-Guerrero, J.J.; Villalobos-Molina, R.; Mata, R. Endothelium-independent relaxation of aorta rings by two stilbenoids from the orchids Scaphyglottis livida. Fitoterapia 2006, 77, 236–239. [Google Scholar] [CrossRef]
- He, W.J.; Fang, T.H.; Ma, X.; Zhang, K.; Ma, Z.Z.; Tu, P.F. Echinacoside elicits endothelium-dependent relaxation in rat aortic rings via an NO-cGMP pathway. Planta Med. 2009, 75, 1400–1404. [Google Scholar] [CrossRef]
- Yılmaz, B.; Usta, C. Ellagic acid-induced endothelium-dependent and endothelium-independent vasorelaxation in rat thoracic aortic rings and the underlying mechanism. Phytother. Res. 2012. [Google Scholar] [CrossRef]
- Huang, H.C.; Lee, C.R.; Chao, P.D.; Chen, C.C.; Chu, S.H. Vasorelaxant effect of emodin, an anthraquinone from a Chinese herb. Eur. J. Pharmacol. 1991, 205, 289–294. [Google Scholar] [CrossRef]
- Martinsen, A.; Baccelli, C.; Navarro, I.; Abad, A.; Quetin-Leclercq, J.; Morel, N. Vascular activity of a natural diterpene isolated from Croton zambesicus and of a structurally similar synthetic trachylobane. Vascul. Pharmacol. 2010, 52, 63–69. [Google Scholar] [CrossRef]
- Hipólito, U.V.; Rodrigues, G.J.; Lunardi, C.N.; Bonaventura, D.; Ambrosio, S.R.; de Oliveira, A.M.; Bendhack, L.M.; da Costa, F.B.; Tirapelli, C.R. Mechanisms underlying the vasorelaxant action of the pimarane ent-8(14),15-pimaradien-3beta-ol in the isolated rat aorta. Eur. J. Pharmacol. 2009, 616, 183–191. [Google Scholar] [CrossRef]
- Aggio, A.; Grassi, D.; Onori, E.; D'Alessandro, A.; Masedu, F.; Valenti, M.; Ferri, C. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur. J. Nutr. 2013, 52, 263–272. [Google Scholar] [CrossRef]
- Cruz, A.J.; Lemos, V.S.; dos Santos, M.H.; Nagem, T.J.; Cortes, S.F. Vascular effects of 7-epiclusianone, a prenylated benzophenone from Rheedia gardneriana, on the rat aorta. Phytomedicine 2006, 13, 442–445. [Google Scholar] [CrossRef]
- Joy, S.; Siow, R.C.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006, 281, 27335–27345. [Google Scholar] [CrossRef]
- Sánchez de Rojas, R.; Somoza, B.; Ortega, T.; Villar, A.M.; Tejerina, T. Vasodilatory effect in rat aorta of eriodictyol obtained from Satureja obovata. Planta Med. 1999, 65, 234–238. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Herrera, M.D.; Perona, J.S.; Ruiz-Gutiérrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br. J. Nutr. 2004, 92, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Raimundo, J.M.; Trindade, A.P.; Velozo, L.S.; Kaplan, M.A.; Sudo, R.T.; Zapata-Sudo, G. The lignan eudesmin extracted from Piper truncatum induced vascular relaxation via activation of endothelial histamine H1 receptors. Eur. J. Pharmacol. 2009, 606, 150–154. [Google Scholar] [CrossRef]
- Interaminense, L.F.; Jucá, D.M.; Magalhães, P.J.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Pharmacological evidence of calcium-channel blockade by essential oil of Ocimum gratissimum and its main constituent, eugenol, in isolated aortic rings from DOCA-salt hypertensive rats. Fundam. Clin. Pharmacol. 2007, 21, 497–506. [Google Scholar] [CrossRef]
- Damiani, C.E.; Rossoni, L.V.; Vassallo, D.V. Vasorelaxant effects of eugenol on rat thoracic aorta. Vascul. Pharmacol. 2003, 40, 59–66. [Google Scholar] [CrossRef]
- Criddle, D.N.; Madeira, S.V.; Soares de Moura, R. Endothelium-dependent and -independent vasodilator effects of eugenol in the rat mesenteric vascular bed. J. Pharm. Pharmacol. 2003, 55, 359–365. [Google Scholar] [CrossRef]
- Fang, L.H.; Mu, Y.M.; Lin, L.L.; Xiao, P.G.; Du, G.H. Vasorelaxant effect of euxanthone in the rat thoracic aorta. Vascul. Pharmacol. 2006, 45, 96–101. [Google Scholar] [CrossRef]
- Yamahara, J.; Kobayashi, G.; Matsuda, H.; Fujimura, H. The vasorelaxant effect of evocarpine in isolated aortic strips: Mode of action. Eur. J. Pharmacol. 1988, 155, 139–143. [Google Scholar] [CrossRef]
- Chiou, W.F.; Chou, C.J.; Shum, A.Y.; Chen, C.F. The vasorelaxant effect of evodiamine in rat isolated mesenteric arteries: Mode of action. Eur. J. Pharmacol. 1992, 215, 277–283. [Google Scholar] [CrossRef]
- Rhyu, M.R.; Kim, J.H.; Kim, E.Y. Radix angelica elicits both nitric oxide-dependent and calcium influx-mediated relaxation in rat aorta. J. Cardiovasc. Pharmacol. 2005, 46, 99–104. [Google Scholar] [CrossRef]
- Rezende, B.A.; Cortes, S.F.; Lemos, V.S. Mechanisms involved in the vasodilator effect of the flavanol floranol in rat small mesenteric arteries. Planta Med. 2004, 70, 465–467. [Google Scholar] [CrossRef]
- Lemos, V.S.; Côrtes, S.F.; dos Santos, M.H.; Ellena, J.; Moreira, M.E.; Doriguetto, A.C. Structure and vasorelaxant activity of floranol, a flavonoid isolated from the roots of Dioclea grandiflora. Chem. Biodivers. 2006, 3, 635–645. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.N.; Wang, S.B.; Wang, S.H.; Du, G.H. Vasorelaxant effect of formononetin in the rat thoracic aorta and its mechanisms. J. Asian. Nat. Prod. Res. 2012, 14, 46–54. [Google Scholar] [CrossRef]
- Iizuka, T.; Sakai, H.; Moriyama, H.; Suto, N.; Nagai, M.; Bagchi, D. Vasorelaxant effects of forsythide isolated from the leaves of Forsythia viridissima on NE-induced aortal contraction. Phytomedicine. 2009, 16, 386–390. [Google Scholar] [CrossRef]
- Morello, S.; Vellecco, V.; Alfieri, A.; Mascolo, N.; Cicala, C. Vasorelaxant effect of the flavonoid galangin on isolated rat thoracic aorta. Life Sci. 2006, 78, 825–830. [Google Scholar] [CrossRef]
- Yuzurihara, M.; Ikarashi, Y.; Goto, K.; Sakakibara, I.; Hayakawa, T.; Sasaki, H. Geissoschizine methyl ether, an indole alkaloid extracted from Uncariae Ramulus et Uncus, is a potent vasorelaxant of isolated rat aorta. Eur. J. Pharmacol. 2002, 444, 183–189. [Google Scholar] [CrossRef]
- Figtree, G.A.; Griffiths, H.; Lu, Y.Q.; Webb, C.M.; MacLeod, K.; Collins, P. Plant-derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J. Am. Coll. Cardiol. 2000, 35, 1977–1985. [Google Scholar] [CrossRef]
- Speroni, F.; Rebolledo, A.; Salemme, S.; Roldán-Palomo, R.; Rimorini, L.; Añón, M.C.; Spinillo, A.; Tanzi, F.; Milesi, V. Genistein effects on Ca2+ handling in human umbilical artery: Inhibition of sarcoplasmic reticulum Ca2+ release and of voltage-operated Ca2+ channels. J. Physiol. Biochem. 2009, 65, 113–124. [Google Scholar] [CrossRef]
- Kim, N.D.; Kang, S.Y.; Park, J.H.; Schini-Kerth, V.B. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur. J. Pharmacol. 1999, 367, 41–49. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, S.J.; Yun, M.R.; Seo, K.W.; Bae, S.S.; Park, J.W.; Lee, Y.J.; Shin, W.J.; Choi, Y.W.; Kim, C.D. Gomisin A from Schisandra chinensis induces endothelium-dependent and direct relaxation in rat thoracic aorta. Planta Med. 2007, 73, 1537–1542. [Google Scholar] [CrossRef]
- Rendón-Vallejo, P.; Hernández-Abreu, O.; Vergara-Galicia, J.; Millán-Pacheco, C.; Mejía, A.; Ibarra-Barajas, M.; Estrada-Soto, S. Ex vivo study of the vasorelaxant activity induced by phenanthrene derivatives isolated from Maxillaria densa. J. Nat. Prod. 2012, 75, 2241–2245. [Google Scholar] [CrossRef]
- Berrougui, H.; Martín-Cordero, C.; Khalil, A.; Hmamouchi, M.; Ettaib, A.; Marhuenda, E.; Herrera, M.D. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol. Res. 2006, 54, 150–157. [Google Scholar] [CrossRef]
- Shi, C.C.; Chen, S.Y.; Wang, G.J.; Liao, J.F.; Chen, C.F. Vasorelaxant effect of harman. Eur. J. Pharmacol. 2000, 390, 319–325. [Google Scholar]
- Xie, Y.W.; Ming, D.S.; Xu, H.X.; Dong, H.; But, P.P. Vasorelaxing effects of Caesalpinia sappan involvement of endogenous nitric oxide. Life Sci. 2000, 67, 1913–1918. [Google Scholar] [CrossRef]
- Orallo, F.; Alvarez, E.; Basaran, H.; Lugnier, C. Comparative study of the vasorelaxant activity, superoxide-scavenging ability and cyclic nucleotide phosphodiesterase-inhibitory effects of hesperetin and hesperidin. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 452–463. [Google Scholar] [CrossRef]
- Xu, H.B.; Huang, Z.Q. Icariin enhances endothelial nitric-oxide synthase expression on human endothelial cells in vitro. Vascul. Pharmacol. 2007, 47, 18–24. [Google Scholar] [CrossRef]
- Xu, H.B.; Huang, Z.Q. Vasorelaxant effects of icariin on isolated canine coronary artery. J. Cardiovasc. Pharmacol. 2007, 49, 207–213. [Google Scholar] [CrossRef]
- He, J.Y.; Zhang, W.; He, L.C.; Cao, Y.X. Imperatorin induces vasodilatation possibly via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Eur. J. Pharmacol. 2007, 573, 170–175. [Google Scholar] [CrossRef]
- Nie, H.; Meng, L.Z.; Zhou, J.Y.; Fan, X.F.; Luo, Y.; Zhang, G.W. Imperatorin is responsible for the vasodilatation activity of Angelica Dahurica var. Formosana regulated by nitric oxide in an endothelium-dependent manner. Chin. J. Integr. Med 2009, 15, 442–447. [Google Scholar] [CrossRef]
- Yu, S.M.; Kuo, S.C. Vasorelaxant effect of isoliquiritigenin, a novel soluble guanylate cyclase activator, in rat aorta. Br. J. Pharmacol. 1995, 114, 1587–1594. [Google Scholar] [CrossRef]
- Pérez-Vizcaíno, F.; Ibarra, M.; Cogolludo, A.L.; Duarte, J.; Zaragozá-Arnáez, F.; Moreno, L.; López-López, G.; Tamargo, J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Exp. Ther. 2002, 302, 66–72. [Google Scholar] [CrossRef]
- Zhang, W.B.; Chen, C.X.; Sim, S.M.; Kwan, C.Y. In vitro vasodilator mechanisms of the indole alkaloids rhynchophylline and isorhynchophylline, isolated from the hook of Uncaria rhynchophylla (Miquel). Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 232–238. [Google Scholar] [CrossRef]
- Wang, G.J.; Wu, X.C.; Lin, Y.L.; Ren, J.; Shum, A.Y.; Wu, Y.Y.; Chen, C.F. Ca2+ channel blocking effect of iso-S-petasin in rat aortic smooth muscle cells. Eur. J. Pharmacol. 2002, 445, 239–245. [Google Scholar] [CrossRef]
- Mendes, L.J.; Capettini, L.S.; Lôbo, L.T.; da Silva, G.A.; Arruda, M.S.; Lemos, V.S.; Côrtes, S.F. Endothelial nitric oxide-dependent vasorelaxant effect of isotirumalin, a dihydroflavonol from Derris urucu, on the rat aorta. Biol. Pharm. Bull. 2011, 34, 1499–1500. [Google Scholar] [CrossRef]
- Duarte, D.F.; Sant'Ana, A.E.; Calixto, J.B. Analysis of the vasorelaxant action of jatrophone in the isolated aorta of the rat: Influence of potassium channel blockers. Eur. J. Pharmacol. 1992, 215, 75–81. [Google Scholar] [CrossRef]
- Silva, A.M.; Brum, R.L.; Calixto, J.B. The relaxant action of jatrophone in rat portal vein. A comparison with protein kinase C inhibitors. Life Sci. 1995, 57, 863–871. [Google Scholar] [CrossRef]
- Xu, Y.C.; Yeung, D.K.; Man, R.Y.; Leung, S.W. Kaempferol enhances endothelium-independent and dependent relaxation in the porcine coronary artery. Mol. Cell. Biochem. 2006, 287, 61–67. [Google Scholar] [CrossRef]
- Padilla, E.; Ruiz, E.; Redondo, S.; Gordillo-Moscoso, A.; Slowing, K.; Tejerina, T. Relationship between vasodilation capacity and phenolic content of Spanish wines. Eur. J. Pharmacol. 2005, 517, 84–91. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; da Costa, F.B.; Coutinho, S.T.; de Oliveira, D.C.; de Oliveira, A.M. Analysis of the mechanisms underlying the vasorelaxant action of kaurenoic acid in the isolated rat aorta. Eur. J. Pharmacol. 2004, 492, 233–241. [Google Scholar] [CrossRef]
- Zamblé, A.; Martin-Nizard, F.; Sahpaz, S.; Reynaert, M.L.; Staels, B.; Bordet, R.; Duriez, P.; Gressier, B.; Bailleul, F. Effects of Microdesmis keayana alkaloids on vascular parameters of erectile dysfunction. Phytother. Res. 2009, 23, 892–895. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Medeiros, I.A. Endothelium-independent vasodilation induced by kolaviron, a biflavonoid complex from Garcinia kola seeds, in rat superior mesenteric arteries. J. Smooth Muscle Res. 2009, 45, 39–53. [Google Scholar] [CrossRef]
- De Oliveira, A.P.; Furtado, F.F.; da Silva, M.S.; Tavares, J.F.; Mafra, R.A.; Araújo, D.A.; Cruz, J.S.; de Medeiros, I.A. Calcium channel blockade as a target for the cardiovascular effects induced by the 8 (17), 12E, 14-labdatrien-18-oic acid (labdane-302). Vascul. Pharmacol. 2006, 44, 338–344. [Google Scholar] [CrossRef]
- Lahlou, S.; de Barros Correia, C.A.; Vasconcelos Dos Santos, M.; David, J.M.; David, J.P.; Duarte, G.P.; Magalhães, P.J. Mechanisms underlying the cardiovascular effects of a labdenic diterpene isolated from Moldenhawera nutans in normotensive rats. Vascul. Pharmacol. 2007, 46, 60–66. [Google Scholar] [CrossRef]
- Assreuy, A.M.; Pinto, N.V.; Lima Mota, M.R.; Passos Meireles, A.V.; Cajazeiras, J.B.; Nobre, C.B.; Soares, P.M.; Cavada, B.S. Vascular smooth muscle relaxation by a lectin from Pisum arvense: Evidences of endothelial NOS pathway. Protein Pept. Lett. 2011, 18, 1107–1111. [Google Scholar] [CrossRef]
- Chen, C.X.; Kwan, C.Y. Endothelium-independent vasorelaxation by leonurine, a plant alkaloid purified from Chinese motherwort (Leonurus artemisiae). Life Sci. 2001, 68, 953–960. [Google Scholar] [CrossRef]
- Andriambeloson, E.; Kleschyov, A.L.; Muller, B.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997, 120, 1053–1058. [Google Scholar] [CrossRef]
- Cao, Y.X.; Zhang, W.; He, J.Y.; He, L.C.; Xu, C.B. Ligustilide induces vasodilatation via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Vascul. Pharmacol. 2006, 45, 171–176. [Google Scholar] [CrossRef]
- Chan, S.S.; Cheng, T.Y.; Lin, G. Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta. J. Ethnopharmacol. 2007, 111, 677–680. [Google Scholar] [CrossRef]
- Jiang, H.; Xia, Q.; Wang, X.; Song, J.; Bruce, I.C. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Pharmazie 2005, 60, 444–447. [Google Scholar]
- Oh, K.S.; Choi, Y.H.; Ryu, S.Y.; Oh, B.K.; Seo, H.W.; Yon, G.H.; Kim, Y.S.; Lee, B.H. Cardiovascular effects of lignans isolated from Saururus chinensis. Planta Med. 2008, 74, 233–238. [Google Scholar]
- El-Bardai, S.; Wibo, M.; Hamaide, M.C.; Lyoussi, B.; Quetin-Leclercq, J.; Morel, N. Characterization of marrubenol, a diterpene extracted from Marrubium vulgare, as an L-type calcium channel blocker. Br. J. Pharmacol. 2003, 140, 1211–1216. [Google Scholar] [CrossRef]
- Khan, A.U.; Ullah, R.; Khan, A.; Mustafa, M.R.; Hussain, J.; Murugan, D.D.; Hadi, A.H. Vasodilator effect of Phlomis bracteosa constituents is mediated through dual endothelium-dependent and endothelium-independent pathways. Clin. Exp. Hypertens. 2012, 34, 132–139. [Google Scholar] [CrossRef]
- Iizuka, T.; Moriyama, H.; Nagai, M. Vasorelaxant effects of methyl brevifolincarboxylate from the leaves of Phyllanthus niruri. Biol. Pharm. Bull. 2006, 29, 177–179. [Google Scholar] [CrossRef]
- Yoo, M.Y.; Lee, B.H.; Choi, Y.H.; Lee, J.W.; Seo, J.H.; Oh, K.S.; Koo, H.N.; Seo, H.W.; Yon, G.H.; Kwon, D.Y.; et al. Vasorelaxant effect of the rootbark extract of Paeonia moutan on isolated rat thoracic aorta. Planta Med. 2006, 72, 1338–1341. [Google Scholar] [CrossRef]
- Cavalcante, H.M.; Ribeiro, T.P.; Silva, D.F.; Nunes, X.P.; Barbosa-Filho, J.M.; Diniz, M.F.; Correia, N.A.; Braga, V.A.; Medeiros, I.A. Cardiovascular effects elicited by milonine, a new 8,14-dihydromorphinandienone alkaloid. Basic Clin. Pharmacol. Toxicol. 2011, 108, 122–130. [Google Scholar] [CrossRef]
- Ojewole, J.A. Cardiovascular effects of mollic acid glucoside, a 1alpha-hydroxycycloartenoid saponin extractive from Combretum molleR Br ex G Don (Combretaceae) leaf. Cardiovasc. J. Afr. 2008, 19, 128–134. [Google Scholar]
- Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Navarrete-Vazquez, G.; Hidalgo-Figueroa, S.; Hernández-Abreu, O.; Estrada-Soto, S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: Ex vivo and in silico studies. Fitoterapia 2012, 83, 1023–1029. [Google Scholar] [CrossRef]
- Orallo, F.; Alzueta, A.F. Preliminary study of the vasorelaxant effects of (+)-nantenine, an alkaloid isolated from Platycapnos spicata, in rat aorta. Planta Med. 2001, 67, 800–806. [Google Scholar] [CrossRef]
- Brito, T.S.; Lima, F.J.; Aragão, K.S.; de Siqueira, R.J.; Sousa, P.J.; Maia, J.G.; Filho, J.D.; Lahlou, S.; Magalhães, P.J. The vasorelaxant effects of 1-nitro-2-phenylethane involve stimulation of the soluble guanylate cyclase-cGMP pathway. Biochem. Pharmacol. 2013, 85, 780–788. [Google Scholar] [CrossRef]
- Ko, F.N.; Lin, C.N.; Liou, S.S.; Huang, T.F.; Teng, C.M. Vasorelaxation of rat thoracic aorta caused by norathyriol isolated from Gentianaceae. Eur. J. Pharmacol. 1991, 192, 133–139. [Google Scholar] [CrossRef]
- Guedes, D.N.; Barbosa-Filho, J.M.; Lemos, V.S.; Côrtes, S.F. Mechanism of the vasodilator effect of 12-O-methylcurine in rat aortic rings. J. Pharm. Pharmacol. 2002, 54, 853–858. [Google Scholar] [CrossRef]
- Fu, X.C.; Wang, M.W.; Li, S.P.; Zhang, Y.; Wang, H.L. Vasodilatation produced by orientin and its mechanism study. Biol. Pharm. Bull. 2005, 28, 37–41. [Google Scholar] [CrossRef]
- Ko, F.N.; Wu, T.S.; Liou, M.J.; Huang, T.F.; Teng, C.M. Vasorelaxation of rat thoracic aorta caused by osthole isolated from Angelica pubescens. Eur. J. Pharmacol. 1992, 219, 29–34. [Google Scholar] [CrossRef]
- Kang, D.G.; Moon, M.K.; Choi, D.H.; Lee, J.K.; Kwon, T.O.; Lee, H.S. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) via a nitric oxide-cGMP pathway. Eur. J. Pharmacol. 2005, 524, 111–119. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; da Costa, F.B.; de Oliveira, A.M. Evidence for the mechanisms underlying the effects of pimaradienoic acid isolated from the roots of Viguiera arenaria on rat aorta. Pharmacology 2004, 70, 31–38. [Google Scholar] [CrossRef]
- Zhu, X.M.; Fang, L.H.; Li, Y.J.; Du, G.H. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vascul. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- Estrada, O.; González-Guzmán, J.M.; Salazar-Bookaman, M.; Fernández, A.Z.; Cardozo, A.; Alvarado-Castillo, C. Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings. Phytomedicine 2011, 18, 464–469. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Dai, Y.; Kong, L.; Wang, F.; Xu, H.; Lu, D.; Song, J.; Hou, Z. Praeruptorin A enantiomers exert distinct relaxant effects on isolated rat aorta rings dependent on endothelium and nitric oxide synthesis. Chem. Biol. Interact. 2010, 186, 239–246. [Google Scholar] [CrossRef]
- Aldini, G.; Carini, M.; Piccoli, A.; Rossoni, G.; Facino, R.M. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sci. 2003, 73, 2883–2898. [Google Scholar] [CrossRef]
- Matsui, T.; Korematsu, S.; Byun, E.B.; Nishizuka, T.; Ohshima, S.; Kanda, T. Apple procyanidins induced vascular relaxation in isolated rat aorta through NO/cGMP pathway in combination with hyperpolarization by multiple K+ channel activations. Biosci. Biotechnol. Biochem. 2009, 73, 2246–2251. [Google Scholar] [CrossRef]
- Tokoudagba, J.M.; Auger, C.; Bréant, L.; N'gom, S.; Chabert, P.; Idris-Khodja, N.; Gbaguidi, F.; Gbenou, J.; Moudachirou, M.; Lobstein, A.; et al. Procyanidin-rich fractions from Parkia biglobosa (Mimosaceae) leaves cause redox-sensitive endothelium-dependent relaxation involving NO and EDHF in porcine coronary artery. J. Ethnopharmacol. 2010, 132, 246–250. [Google Scholar] [CrossRef]
- Ajay, M.; Gilani, A.U.; Mustafa, M.R. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003, 74, 603–612. [Google Scholar] [CrossRef]
- Chiwororo, W.D.; Ojewole, J.A. Dual effect of quercetin on rat isolated portal vein smooth muscle contractility. Cardiovasc. J. Afr. 2010, 21, 132–136. [Google Scholar]
- Guerrero, M.F.; Puebla, P.; Carrón, R.; Martín, M.L.; San Román, L. Quercetin 3,7-dimethyl ether: A vasorelaxant flavonoid isolated from Croton schiedeanus Schlecht. J. Pharm. Pharmacol. 2002, 54, 1373–1378. [Google Scholar] [CrossRef]
- Fan, Y.F.; Chen, Z.W.; Guo, Y.; Wang, Q.H.; Song, B. Cellular mechanisms underlying hyperin-induced relaxation of rat basilar artery. Fitoterapia 2011, 82, 626–631. [Google Scholar] [CrossRef]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. 1996, 27, 363–366. [Google Scholar] [CrossRef]
- Gojkovic-Bukarica, L.; Novakovic, A.; Kanjuh, V.; Bumbasirevic, M.; Lesic, A.; Heinle, H. A role of ion channels in the endothelium-independent relaxation of rat mesenteric artery induced by resveratrol. J. Pharmacol. Sci. 2008, 108, 124–130. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Nunes, X.P.; Barbosa-Filho, J.M.; Lemos, V.S.; Pinho, J.F.; Roman-Campos, D.; de Medeiros, I.A.; Araújo, D.A.; Cruz, J.S. (S)-reticuline induces vasorelaxation through the blockade of L-type Ca(2+) channels. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 115–125. [Google Scholar] [CrossRef]
- Xia, M.L.; Zhou, X.M.; Yao, H.; Jiang, H.D.; Bruce, I.C.; Wei, E.Q.; Xia, Q. Rutin-induced endothelium-dependent vasorelaxation in rat aortic rings and the underlying mechanism. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 6, 5595–5597. [Google Scholar]
- Lam, F.F.; Yeung, J.H.; Kwan, Y.W.; Chan, K.M.; Or, P.M. Salvianolic acid B, an aqueous component of danshen (Salvia miltiorrhiza), relaxes rat coronary artery by inhibition of calcium channels. Eur. J. Pharmacol. 2006, 553, 240–245. [Google Scholar] [CrossRef]
- Hu, C.M.; Cheng, H.W.; Cheng, Y.W.; Kan, J.J. Mechanisms underlying the induction of vasorelaxation in rat thoracic aorta by sanguinarine. Jpn. J. Pharmacol. 2001, 85, 47–53. [Google Scholar] [CrossRef]
- Sano, S.; Sugiyama, K.; Ito, T.; Katano, Y.; Ishihata, A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011, 59, 6209–6213. [Google Scholar] [CrossRef]
- Pan, Z.; Feng, T.; Shan, L.; Cai, B.; Chu, W.; Niu, H.; Lu, Y.; Yang, B. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother. Res. 2008, 22, 1428–1433. [Google Scholar] [CrossRef]
- Wang, G.J.; Shum, A.Y.; Lin, Y.L.; Liao, J.F.; Wu, X.C.; Ren, J.; Chen, C.F. Calcium channel blockade in vascular smooth muscle cells: Major hypotensive mechanism of S-petasin, a hypotensive sesquiterpene from Petasites formosanus. J. Pharmacol. Exp. Ther. 2001, 297, 240–246. [Google Scholar]
- Kwan, C.Y. Plant-derived drugs acting on cellular Ca2+ mobilization in vascular smooth muscle: Tetramethylpyrazine and tetrandrine. Stem Cells 1994, 12, 64–67. [Google Scholar] [CrossRef]
- Shao, Z.; Li, J.; Zhao, Z.; Gao, C.; Sun, Z.; Liu, X. Effects of tetramethylpyrazine on nitric oxide/cGMP signaling after cerebral vasospasm in rabbits. Brain Res. 2010, 1361, 67–75. [Google Scholar]
- Kim, E.Y.; Kim, J.H.; Rhyu, M.R. Endothelium-independent vasorelaxation by Ligusticum wallichii in isolated rat aorta: Comparison of a butanolic fraction and tetramethylpyrazine, the main active component of Ligusticum wallichii. Biol. Pharm. Bull. 2010, 33, 1360–1363. [Google Scholar] [CrossRef]
- Peng, W.; Hucks, D.; Priest, R.M.; Kan, Y.M.; Ward, J.P. Ligustrazine-induced endothelium-dependent relaxation in pulmonary arteries via an NO-mediated and exogenous L-arginine-dependent mechanism. Br. J. Pharmacol. 1996, 119, 1063–1071. [Google Scholar] [CrossRef]
- Tur, R.; Magraner, J.; Catret, M.; Elorriaga, M.; Ivorra, M.D.; D'Ocon, P.; Bermejo, A.; Cabedo, N.; Cortes, D.; Anselmi, E. Mechanism of vascular relaxation by thaligrisine: Functional and binding assays. Life Sci. 2000, 67, 1535–1548. [Google Scholar] [CrossRef]
- Hernández-Abreu, O.; Castillo-España, P.; León-Rivera, I.; Ibarra-Barajas, M.; Villalobos-Molina, R.; González-Christen, J.; Vergara-Galicia, J.; Estrada-Soto, S. Antihypertensive and vasorelaxant effects of tilianin isolated from Agastache mexicana are mediated by NO/cGMP pathway and potassium channel opening. Biochem. Pharmacol. 2009, 78, 54–61. [Google Scholar]
- Silva, R.M.; Oliveira, F.A.; Cunha, K.M.; Maia, J.L.; Maciel, M.A.; Pinto, A.C.; Nascimento, N.R.; Santos, F.A.; Rao, V.S. Cardiovascular effects of trans-dehydrocrotonin, a diterpene from Croton cajucara in rats. Vascul. Pharmacol. 2005, 43, 11–18. [Google Scholar]
- Orallo, F.; Alvarez, E.; Camiña, M.; Leiro, J.M.; Gómez, E.; Fernández, P. The possible implication of trans-Resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol. Pharmacol. 2002, 61, 294–302. [Google Scholar] [CrossRef]
- Elíes, J.; Cuíñas, A.; García-Morales, V.; Orallo, F.; Campos-Toimil, M. Trans-resveratrol simultaneously increases cytoplasmic Ca(2+) levels and nitric oxide release in human endothelial cells. Mol. Nutr. Food Res. 2011, 55, 1237–1248. [Google Scholar] [CrossRef]
- Matsuo, H.; Okamoto, R.; Zaima, K.; Hirasawa, Y.; Ismail, I.S.; Lajis, N.H.; Morita, H. New vasorelaxant indole alkaloids, villocarines A-D from Uncaria villosa. Bioorg. Med. Chem. 2011, 19, 4075–4079. [Google Scholar] [CrossRef]
- Arai, H.; Zaima, K.; Mitsuta, E.; Tamamoto, H.; Saito, A.; Hirasawa, Y.; Rahman, A.; Kusumawati, I.; Zaini, N.C.; Morita, H. Alstiphyllanines I-O, ajmaline type alkaloids from Alstonia macrophylla showing vasorelaxant activity. Bioorg. Med. Chem. 2012, 20, 3454–3459. [Google Scholar] [CrossRef]
- Duarte, J.; Vallejo, I.; Pérez-Vizcaino, F.; Jiménez, R.; Zarzuelo, A.; Tamargo, J. Effects of visnadine on rat isolated vascular smooth muscles. Planta Med. 1997, 63, 233–236. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Vizcaíno, F.; Torres, A.I.; Zarzuelo, A.; Jiménez, J.; Tamargo, J. Vasodilator effects of visnagin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1995, 286, 115–122. [Google Scholar] [CrossRef]
- Seya, K.; Furukawa, K.; Taniguchi, S.; Kodzuka, G.; Oshima, Y.; Niwa, M.; Motomura, S. Endothelium-dependent vasodilatory effect of vitisin C, a novel plant oligostilbene from Vitis plants (Vitaceae), in rabbit aorta. Clin. Sci. 2003, 105, 73–79. [Google Scholar] [CrossRef]
- Del Valle-Mondragón, L.; Tenorio-López, F.A.; Torres-Narváez, J.C.; Zarco-Olvera, G.; Pastelín-Hernández, G. Coronary vasodilator activity of vulgarenol, a sesquiterpene isolated from Magnolia grandiflora, and its possible mechanism. Phytother. Res. 2009, 23, 666–671. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Kang, J.J. Mechanism of vasorelaxation of thoracic aorta caused by xanthone. Eur. J. Pharmacol. 1997, 336, 23–8. [Google Scholar] [CrossRef]
- Campos, M.G.; Oropeza, M.V.; Villanueva, T.; Aguilar, M.I.; Delgado, G.; Ponce, H.A. Xanthorrhizol induces endothelium-independent relaxation of rat thoracic aorta. Life Sci. 2000, 67, 327–333. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol 2006, 147 (Suppl. 1), S193–S201. [Google Scholar]
- Melikian, N.; Seddon, M.D.; Casadei, B.; Chowienczyk, P.J.; Shah, A.M. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc. Med. 2009, 19, 256–262. [Google Scholar] [CrossRef]
- Shah, M.R.; Wedgwood, S.; Czech, L.; Kim, G.A.; Lakshminrusimha, S.; Schumacker, P.T.; Steinhorn, R.H.; Farrow, K.N. Cyclic stretch induces inducible nitric oxide synthase and soluble guanylate cyclase in pulmonary artery smooth muscle cells. Int. J. Mol. Sci. 2013, 14, 4334–4348. [Google Scholar] [CrossRef]
- Li, H.; Fosterman, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Li, H.; Wallerath, T.; Munzel, T.; Forstermann, U. Regulation of endothelial-type NO synthase expression in pathophisiology and in response to drugs. Nitric Oxide 2002, 7, 149–164. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar]
- Steinkamp-Fenske, K.; Bollinger, L.; Xu, H.; Yao, Y.; Horke, S.; Förstermann, U.; Li, H. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J. Pharmacol. Exp. Ther. 2007, 322, 836–842. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Forstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef]
- Xia, N.; Bollinger, L.; Steinkamp-Fenske, K.; Förstermann, U.; Li, H. Prunella vulgaris L. Upregulates eNOS expression in human endothelial cells. Am. J. Chin. Med. 2010, 38, 599–611. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kawakami, S.; Yanae, K.; Sano, S.; Uchida, H.; Inagaki, H.; Ito, T. Effect of long-term piceatannol treatment on eNOS levels in cultured endothelial cells. Biochem. Biophys. Res. Commun. 2012. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, H.K.; Choi, Y.W.; Lee, Y.J.; Bae, S.S.; Han, J.; Kim, C.D. Gomisin A induces Ca2+-dependent activation of eNOS in human coronary artery endothelial cells. J. Ethnopharmacol. 2009, 125, 291–296. [Google Scholar] [CrossRef]
- Brandes, R.P.; Schmitz-Winnenthal, F.H.; Félétou, M.; Gödecke, A.; Huang, P.L.; Vanhoutte, P.M.; Fleming, I.; Busse, R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 9747–9752. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Lu, X.; Guo, J.; Wu, J.M. Differential regulation of proliferation, cell cycle control and gene expression in cultured human aortic and pulmonary artery endothelial cells by resveratrol. Int. J. Mol. Med. 2010, 26, 743–749. [Google Scholar]
- Bruder, J.L.; Hsieh, T.; Lerea, K.M.; Olson, S.C.; Wu, J.M. Induced cytoskeletal changes in bovine pulmonary artery endothelial cells by resveratrol and the accompanying modified responses to arterial shear stress. BMC Cell Biol. 2001, 2, 10–1186. [Google Scholar]
- Cherkaoui-Tangi, K.; Lachkar, M.; Wibo, M.; Morel, N.; Gilani, A.H.; Lyoussi, B. Pharmacological studies on hypotensive, diuretic and vasodilator activities of chrysin glucoside from Calycotome villosa in rats. Phytother. Res. 2008, 22, 356–361. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamaki, F.; Koike, K.; Toro, L. New insights into the intracellular mechanisms by which PGI2 analogues elicit vascular relaxation: Cyclic AMP-independent, Gs-protein mediated-activation of maxiK Channel. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2004, 2, 257–265. [Google Scholar] [CrossRef]
- Yeung, D.K.; Leung, S.W.; Xu, Y.C.; Vanhoutte, P.M.; Man, R.Y. Puerarin, an isoflavonoid derived from Radix puerariae, potentiates endothelium-independent relaxation via the cyclic AMP pathway in porcine coronary artery. Eur. J. Pharmacol. 2006, 552, 105–111. [Google Scholar] [CrossRef]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef]
- Pauvert, O.; Lugnier, C.; Keravis, T.; Marthan, R.; Rousseau, E.; Savineau, J.P. Effect of sildenafil on cyclic nucleotide phosphodiesterase activity, vascular tone and calcium signaling in rat pulmonary artery. Br. J. Pharmacol. 2003, 139, 513–522. [Google Scholar] [CrossRef]
- Rybalkin, S.D.; Yan, C.; Bornfeldt, K.E.; Beavo, J.A. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ. Res. 2003, 93, 280–291. [Google Scholar] [CrossRef]
- Keravis, T.; Thaseldar-Roumie, R.; Lugnier, C. Assessment of phosphodiesterase isozyme contribution in cell and tissue extracts. Methods Mol. Biol. 2005, 307, 63–74. [Google Scholar]
- Ko, E.A.; Han, J.; Jung, I.D.; Park, W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth Muscle Res. 2008, 44, 65–81. [Google Scholar] [CrossRef]
- Hayabuchi, Y.; Nakaya, Y.; Mawatari, K.; Inoue, M.; Sakata, M.; Kagami, S. Cell membrane stretch activates intermediate-conductance Ca2+-activated K+ channels in arterial smooth muscle cells. Heart Vessels 2011, 26, 91–100. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Ella, S.R.; Davis, M.J.; Braun, A.P. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010, 584, 2033–2042. [Google Scholar]
- Jackson, W.F. Potassium channels in the peripheral microcirculation. Microcirculation 2005, 12, 113–127. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Kaldunski, M.; Jacobs, E.R.; Harder, D.R.; Roman, R.J. Coexistence of two types of Ca (2+)-activated K+ channels in rat renal arterioles. Am. J. Physiol. 1996, 270, F69–F81. [Google Scholar]
- Michelakis, E.D.; Reeve, H.L.; Huang, J.M.; Tolarova, S.; Nelson, D.P.; Weir, E.K.; Archer, S.L. Potassium channel diversity in vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1997, 75, 889–897. [Google Scholar] [CrossRef]
- Hill, C.E.; Phillips, J.K.; Sandow, S.L. Heterogeneous control of blood flow amongst different vascular beds. Med. Res. Rev. 2001, 21, 1–60. [Google Scholar]
- Shao, Y.; Liew, R.; Guan, B.; Wong, P.E.; Shim, W.S.; Chua, Y.; He, G. Different expression of large-conductance calcium-activated K+ channels in human internal mammary and radial arteries. Cardiovasc Res. 2011, 89, 329–335. [Google Scholar] [CrossRef]
- Eichhorn, B.; Dobrev, D. Vascular large conductance calcium-activated potassium channels: functional role and therapeutic potential. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 376, 145–155. [Google Scholar] [CrossRef]
- Félétou, M.; Vanhoutte, P.M. Endothelium-derived hyperpolarizing factor: Where are we now? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1215–1225. [Google Scholar] [CrossRef]
- Cogolludo, A.; Frazziano, G.; Briones, A.M.; Cobeño, L.; Moreno, L.; Lodi, F.; Salaices, M.; Tamargo, J.; Perez-Vizcaino, F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc. Res. 2007, 73, 424–431. [Google Scholar] [CrossRef]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef]
- Dong, K.; Tao, Q.M.; Shan, Q.X.; Jin, H.F.; Pan, G.B.; Chen, J.Z.; Zhu, J.H.; Xia, Q. Endothelium-independent vasorelaxant effect of puerarin on rat thoracic aorta. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 5, 3757–3760. [Google Scholar]
- Li, Y.; Johnson, G.; Romine, J.L.; Meanwell, N.A.; Martin, S.W.; Dworetzky, S.I.; Boissard, C.G.; Gribkoff, V.K.; Starrett, J.E., Jr. Novel openers of Ca2+-dependent large-conductance potassium channels: Symmetrical pharmacophore and electrophysiological evaluation of bisphenols. Bioorg. Med. Chem. Lett. 2003, 13, 1437–1439. [Google Scholar]
- Sandow, S.L.; Grayson, T.H. Limits of isolation and culture: Intact vascular endothelium and BKCa. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1–H7. [Google Scholar] [CrossRef]
- Lam, F.F.; Yeung, J.H.; Chan, K.M.; Or, P.M. Dihydrotanshinone, a lipophilic component of Salvia miltiorrhiza (danshen), relaxes rat coronary artery by inhibition of calcium channels. J. Ethnopharmacol. 2008, 119, 318–321. [Google Scholar] [CrossRef]
- Zholos, A. Pharmacology of transient receptor potential melastatin channels in the vasculature. Br. J. Pharmacol. 2010, 159, 1559–1571. [Google Scholar] [CrossRef]
- Earley, S. TRPA1 channels in the vasculature. Br. J. Pharmacol. 2012, 167, 13–22. [Google Scholar] [CrossRef]
- Earley, S.; Reading, S.; Brayden, J.E. Functional Significance of Transient Receptor Potential Channels in Vascular Function. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 361–376. [Google Scholar]
- Cole, W.C.; Welsh, D.G. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch. Biochem. Biophys. 2011, 510, 160–173. [Google Scholar] [CrossRef]
- Li, L.; Eto, M.; Lee, M.R.; Morita, F.; Yazawa, M.; Kitazawa, T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J. Physiol. 1998, 508, 871–881. [Google Scholar] [CrossRef]
- Nishimura, J.; Khalil, R.A.; Drenth, J.P.; van Breemen, C. Evidence for increased myofilament Ca2+ sensitivity in norepinephrine-activated vascular smooth muscle. Am. J. Physiol. 1990, 259, H2–H8. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.Á. Vasodilator Compounds Derived from Plants and Their Mechanisms of Action. Molecules 2013, 18, 5814-5857. https://doi.org/10.3390/molecules18055814
Luna-Vázquez FJ, Ibarra-Alvarado C, Rojas-Molina A, Rojas-Molina I, Zavala-Sánchez MÁ. Vasodilator Compounds Derived from Plants and Their Mechanisms of Action. Molecules. 2013; 18(5):5814-5857. https://doi.org/10.3390/molecules18055814
Chicago/Turabian StyleLuna-Vázquez, Francisco J., César Ibarra-Alvarado, Alejandra Rojas-Molina, Isela Rojas-Molina, and Miguel Ángel Zavala-Sánchez. 2013. "Vasodilator Compounds Derived from Plants and Their Mechanisms of Action" Molecules 18, no. 5: 5814-5857. https://doi.org/10.3390/molecules18055814