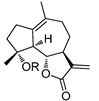
Synthesis of Micheliolide Derivatives and Their Activities against AML Progenitor Cells
Abstract
:1. Introduction
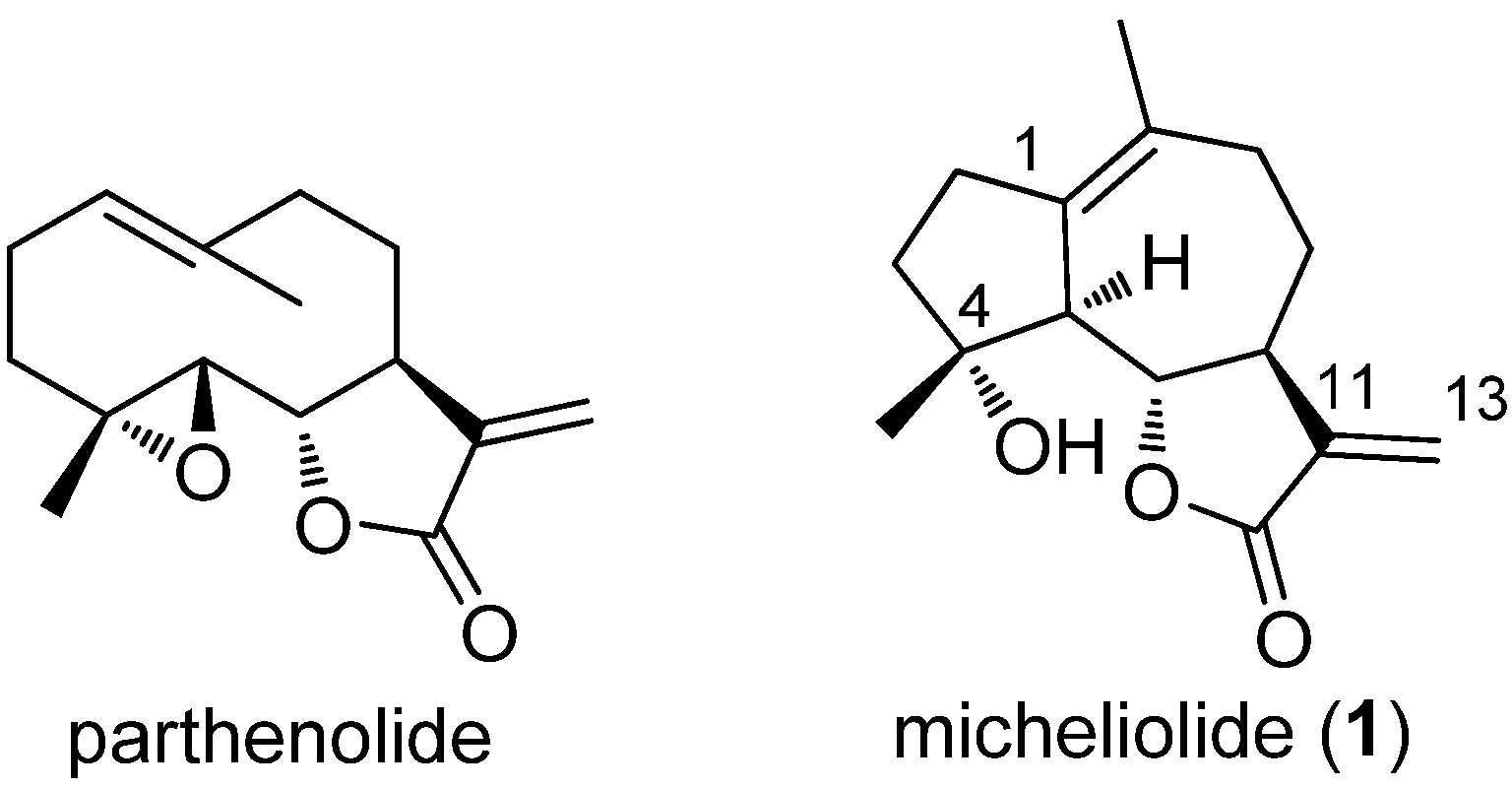
2. Results and Discussion
2.1. Chemistry
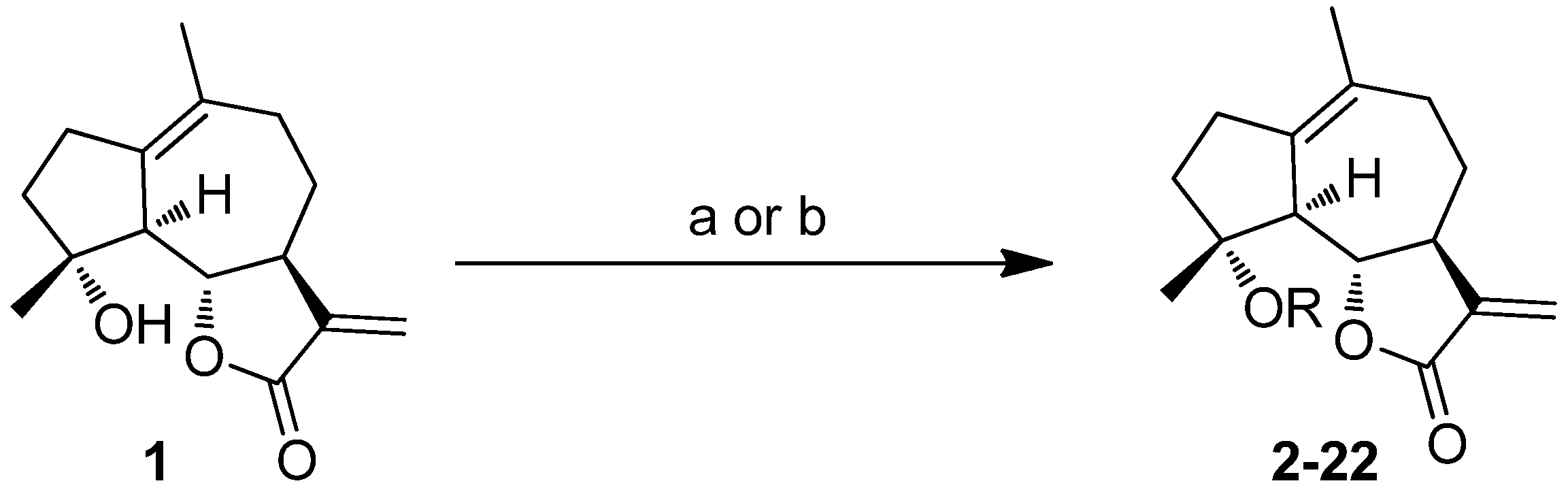
2.2. Activities against AML Cell Lines
| Compounds | R | IC50b (µM) | Times f | ||
|---|---|---|---|---|---|
| HL-60 c | HL-60/A d | KG-1a e | |||
| DOX g | - | 0.05 ± 0.01 | 6.7 ± 1.1 | 1.0 ± 0.3 | 20 |
| 1 (MCL) | H | 5.5 ± 1.4 h | 6.2 ± 2.2 h | 13.4 ± 1.0 | 2.4 |
| 2 h |  | 9.9 ± 0.9 | 10.2 ± 0.1 | - | - |
| 3 |  | 16.7 ± 0.8 | 18.9 ± 4.6 | - | - |
| 4 |  | 3.5 ± 0.6 | 6.2 ± 0.4 | - | - |
| 5 | 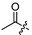 | 12.0 ± 3.2 | 8.3 ± 2.3 | - | - |
| 6 | 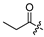 | 10.0 ± 0.6 | 15.6 ± 0.1 | - | - |
| 7 | 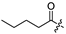 | 11.8 ± 1.4 | 14.2 ± 2.2 | 15.9 ± 0.9 | 1.3 |
| 8 |  | 13.7 ± 1.7 | 22.0 ± 1.4 | - | - |
| 9 | 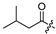 | 10.1 ± 2.2 | 12.5 ± 0.3 | 11.5 ± 1.4 | 1.1 |
| 10 | 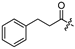 | 15.7 ± 1.7 | 15.1 ± 4.2 | - | - |
| 11 | 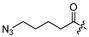 | 16.1 ± 3.1 | 29.7 ± 5.9 | - | - |
| 12 |  | 12.4 ± 0.2 | 18.0 ± 1.0 | - | - |
| 13 |  | 7.2 ± 2.7 | 15.7 ± 3.0 | 19.4 ± 4.2 | 2.7 |
| 14 | 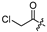 | 16.1 ± 1.9 | 20.3 ± 5.7 | - | - |
| 15 |  | 18.0 ± 3.4 | 20.0 ± 3.2 | - | - |
| 16 |  | 2.8 ± 0.9 | 4.2 ± 0.2 | 7.5 ± 0.9 | 2.7 |
| 17 |  | 13.7 ± 2.6 | 16.7 ± 1.0 | - | - |
| 18 |  | 7.4 ± 1.6 | 8.5 ± 1.8 | 10.4 ± 2.3 | 1.4 |
| 19 | 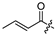 | 12.6 ± 0.2 | 11.7 ± 2.2 | - | - |
| 20 |  | 13.1 ± 1.2 | 17.6 ± 4.6 | - | - |
| 21 | 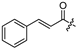 | 15.1 ± 1.9 | 14.7 ± 1.9 | - | - |
| 22 | 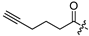 | 8.1 ± 1.4 | 10.8 ± 1.9 | 10.5 ± 0.6 | 1.3 |
| 23 | 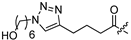 | 16.4 ± 4.3 | 29.0 ± 1.4 | - | - |
3. Experimental
3.1. General
3.2. Product Synthesis and Characterization
General Procedure for the Synthesis of Compounds 5, 6, 9, 10, 13, and 18–22
General Procedure for the Synthesis of Compounds 7, 8, and 14–16
General Procedure for the Synthesis of Compounds 11 and 12
Synthesis of (3aS,9R,9aS,9bS)-6,9-Dimethyl-3-methylene-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl 5-bromopentanoate (17)
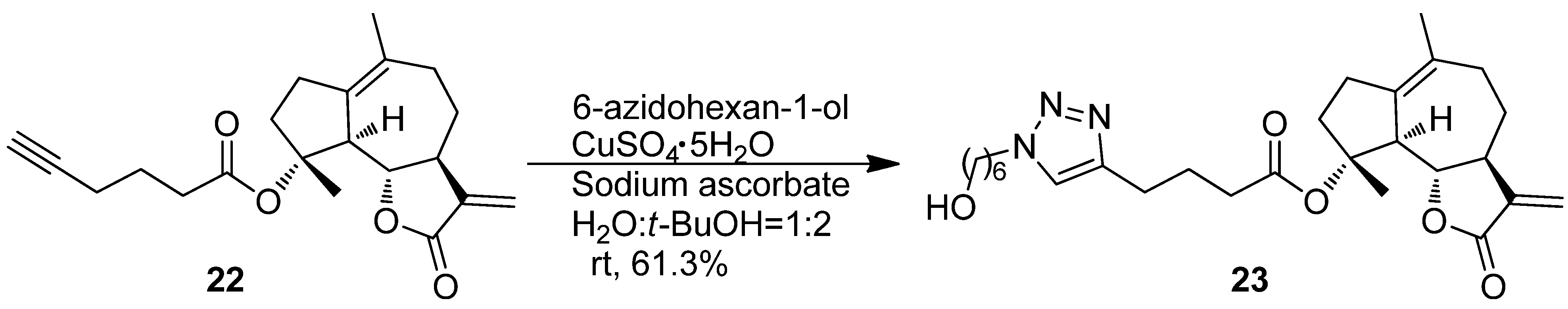
Synthesis of (3aS,9R,9aS,9bS)-6,9-Dimethyl-3-methylene-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl 4-(1-(hydroxymethyl)-1H-1,2,3-triazol-4-yl)butanoate (23)
3.3. Biological assay for Activity of Compounds 1–23
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Barth, B.M.; Altinoğlu, E.I.; Shanmugavelandy, S.S.; Kaiser, J.M.; Crespo-Gonzalez, D.; DiVittore, N.A.; McGovern, C.; Goff, T.M.; Keasey, N.R.; Adair, J.H.; et al. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano 2011, 5, 5325–5327. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Blair, A.; Hogge, D.E.; Sutherland, H.J. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34+/CD71−/HLA-DR−. Blood 1998, 92, 4325–4335. [Google Scholar]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- She, M.; Niu, X.; Chen, X.; Li, J.; Zhou, M.; He, Y.; Le, Y.; Guo, K. Resistance of leukemic stem-cells in AML cell line KG1a to natural killer cell-mediated cytotoxicity. Cancer Lett. 2012, 318, 173–179. [Google Scholar] [CrossRef]
- Jawad, M.; Seedhouse, C.; Mony, U.; Grundy, M.; Russell, N.H.; Pallis, M. Analysis of factors that affect in vitro chemosensitivity of leukaemic stem and progenitor cells to gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukaemia sensitivity to mylotarg by stem cells in AML. Leukemia 2010, 24, 74–80. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4427–4435. [Google Scholar] [CrossRef]
- Neelakantan, S.; Nasim, S.; Guzman, M.L.; Jordan, C.T.; Crooks, P.A. Aminoparthenolides as novel anti-leukemic agents: Discovery of the NF-kappaB inhibitor, DMAPT (LC-1). Bioorg. Med. Chem. Lett. 2009, 19, 4346–4349. [Google Scholar] [CrossRef]
- Kevin, P. New agents for the treatment of leukemia: Discovery of DMAPT (LC-1). Drug Discov. Today 2010, 15, 322. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
- Schall, A.; Reiser, O. Synthesis of biologically active guaianolides with a trans-annulated lactone moiety. Eur. J. Org. Chem. 2008, 2353–2364. [Google Scholar] [CrossRef]
- Ogura, M.; Cordell, G.A.; Farnsworth, N.R. Anticancer sesquiterpene lactones of Michelia compressa (magnoliaceae). Phytochemistry 1978, 17, 957–961. [Google Scholar] [CrossRef]
- Jacobsson, U.; Kumar, V.; Saminathan, S. Sesquiterpene lactones from Michelia champaca. Phytochemistry 1995, 39, 839–843. [Google Scholar] [CrossRef]
- Zhai, J.-D.; Li, D.; Long, J.; Zhang, H.-L.; Lin, J.-P.; Qiu, C.-J.; Zhang, Q.; Chen, Y. Biomimetic semisynthesis of arglabin from parthenolide. J. Org. Chem. 2012, 77, 7103–7107. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Eakin, M.A.; Thomas, A.M. Tumor inhibitors. 69. Structure-cytotoxicity relations among the sesquiterpene lactones. J. Med. Chem. 1971, 14, 1147–1152. [Google Scholar] [CrossRef]
- Lee, K.-H.; Meck, R.; Piantadosi, C.; Huang, E.-S. Antitumor agents. 4. Cytotoxicity and in vivo activity of helenalin esters and related derivatives. J. Med. Chem. 1973, 16, 299–301. [Google Scholar] [CrossRef]
- Zhao, H.; Pendri, A.; Greenwald, R.B. General procedure for acylation of 3° alcohols: Scandium triflate/DMAP reagent. J. Org. Chem. 1998, 63, 7559–7562. [Google Scholar] [CrossRef]
- Banker, D.E.; Cooper, J.J.; Fennell, D.A.; Willman, C.L.; Appelbaum, F.R.; Cotter, F.E. PK11195, a peripheral benzodiazepine receptor ligand, chemosensitizes acute myeloid leukemia cells to relevant therapeutic agents by more than one mechanis. Leukemia Res. 2002, 26, 91–106. [Google Scholar] [CrossRef]
- Soligo, D.; Servida, F.; Delia, D.; Fontanella, E.; Lamorte, G.; Caneva, L.; Fumiatti, R.; Deliliers, G.L. The apoptogenic response of human myeloid leukaemia cell lines and of normal and malignant haematopoietic progenitor cells to the proteasome inhibitor PSI. Br. J. Haematol. 2001, 113, 126–135. [Google Scholar] [CrossRef]
- Bailly, J.D.; Muller, C.; Jaffrézou, J.P.; Demur, C.; Gassar, G.; Bordier, C.; Laurent, G. Lack of correlation between expression and function of P-glycoprotein in acute myeloid leukemia cell lines. Leukemia 1995, 9, 799–807. [Google Scholar]
- Sample Availability: Samples of all compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, W.-W.; Shi, Q.-Q.; Ding, Y.-H.; Long, J.; Zhang, Q.; Chen, Y. Synthesis of Micheliolide Derivatives and Their Activities against AML Progenitor Cells. Molecules 2013, 18, 5980-5992. https://doi.org/10.3390/molecules18055980
Ma W-W, Shi Q-Q, Ding Y-H, Long J, Zhang Q, Chen Y. Synthesis of Micheliolide Derivatives and Their Activities against AML Progenitor Cells. Molecules. 2013; 18(5):5980-5992. https://doi.org/10.3390/molecules18055980
Chicago/Turabian StyleMa, Wei-Wei, Qian-Qian Shi, Ya-Hui Ding, Jing Long, Quan Zhang, and Yue Chen. 2013. "Synthesis of Micheliolide Derivatives and Their Activities against AML Progenitor Cells" Molecules 18, no. 5: 5980-5992. https://doi.org/10.3390/molecules18055980