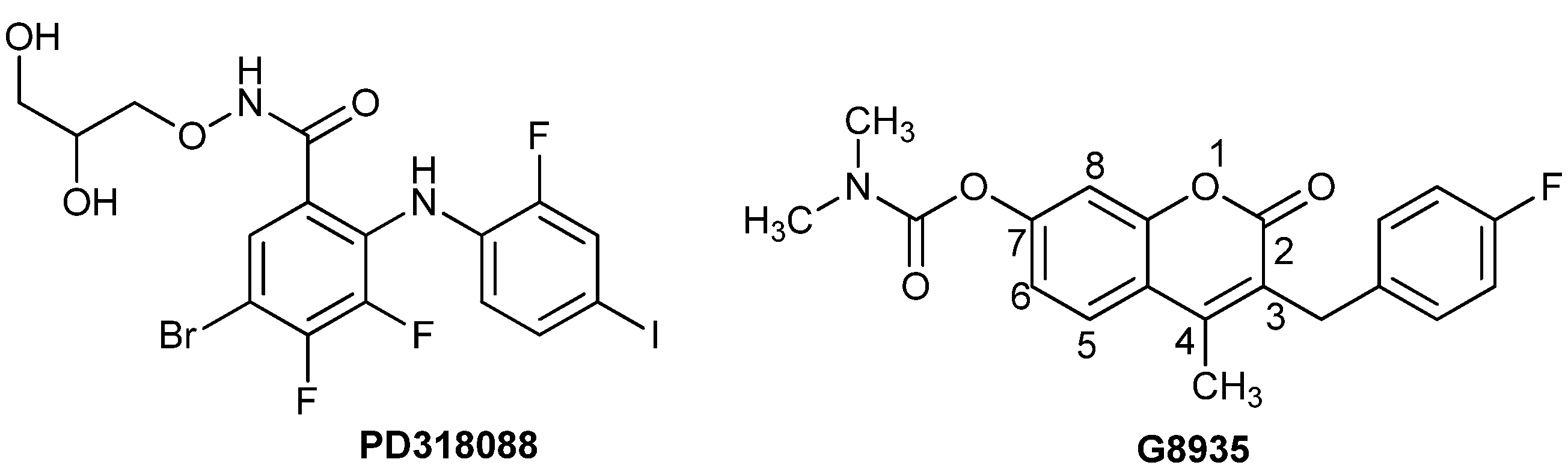
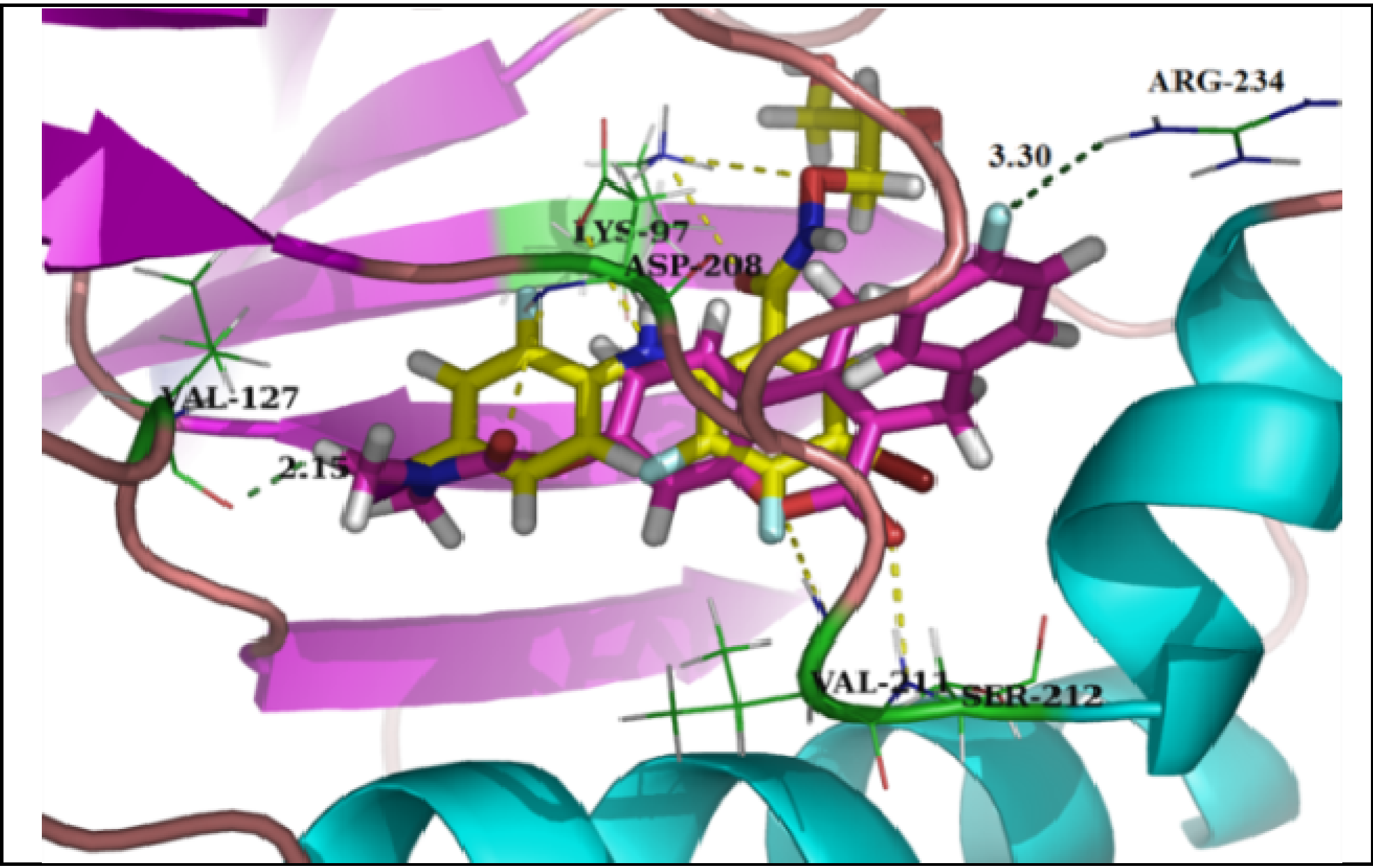
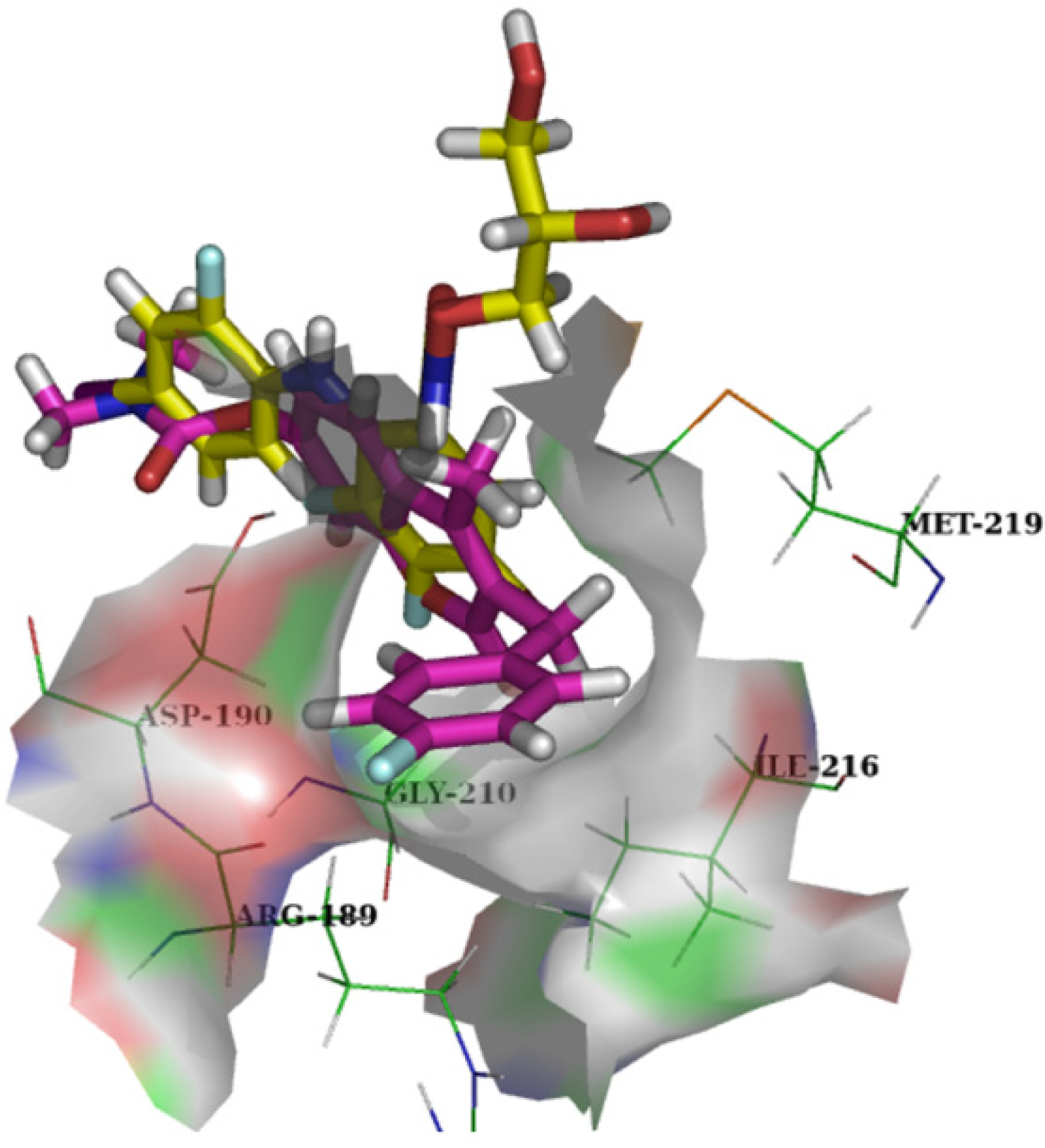
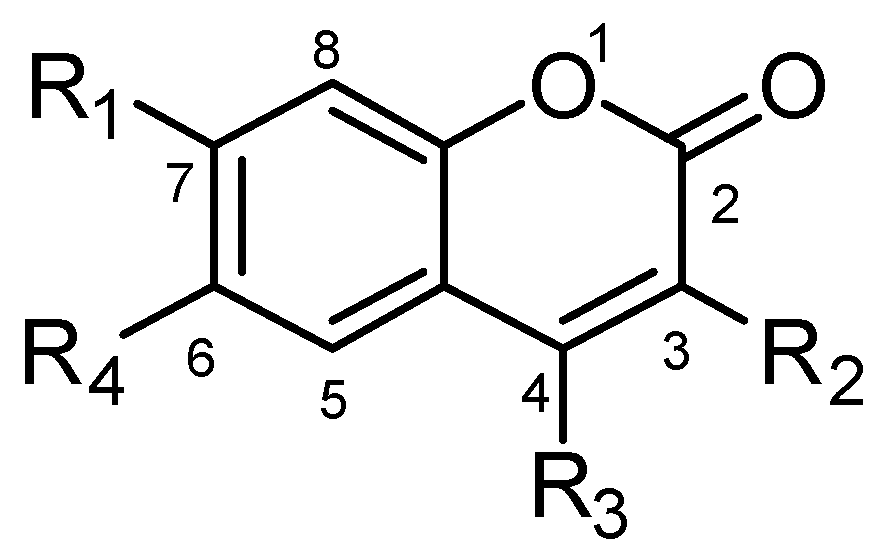
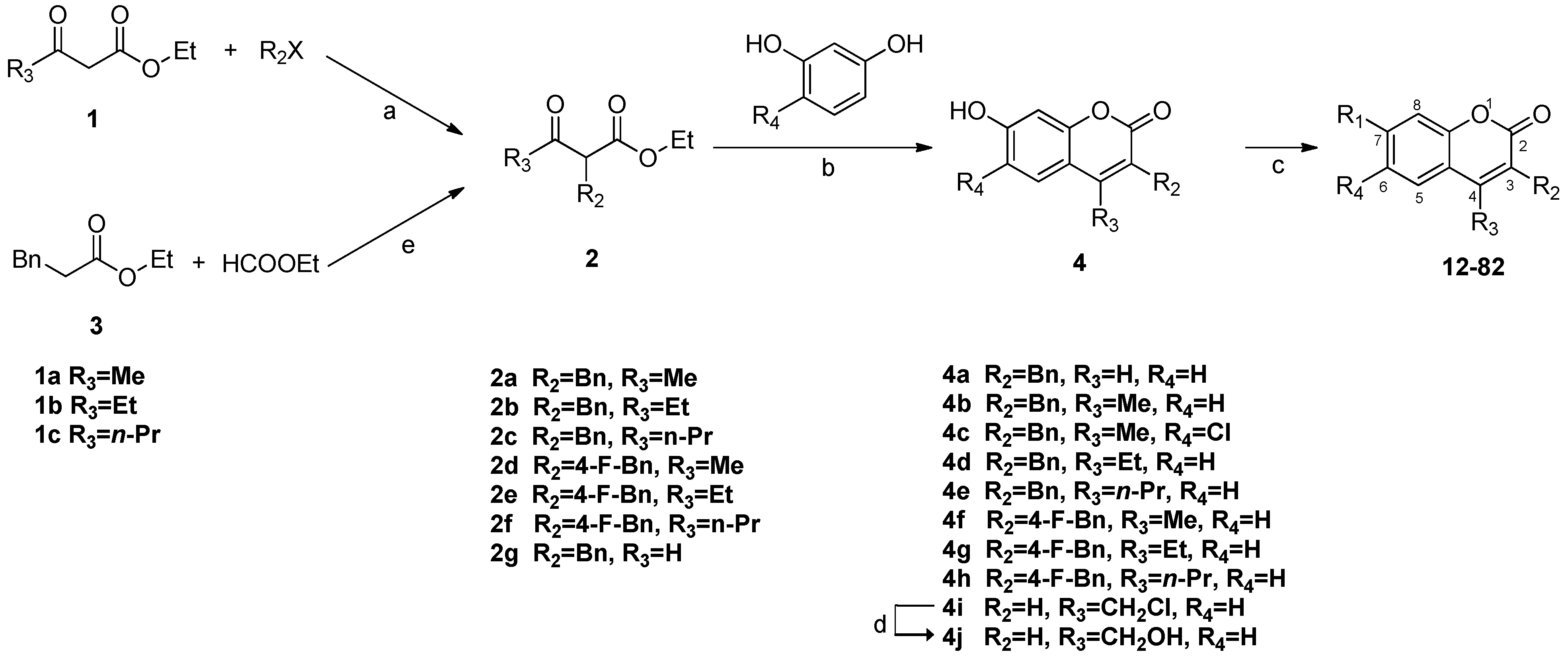
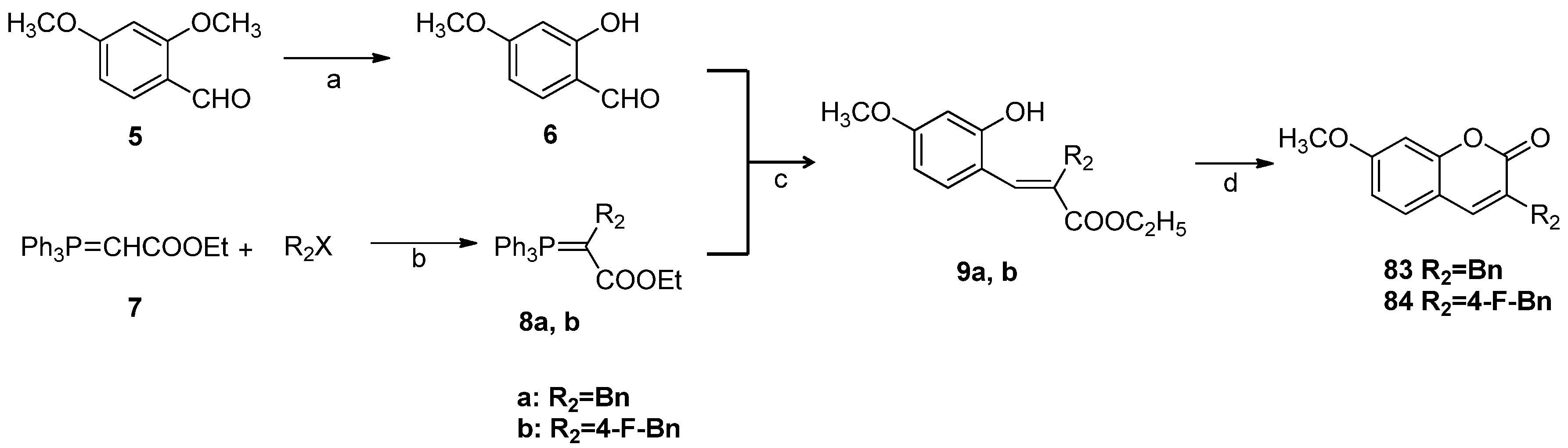
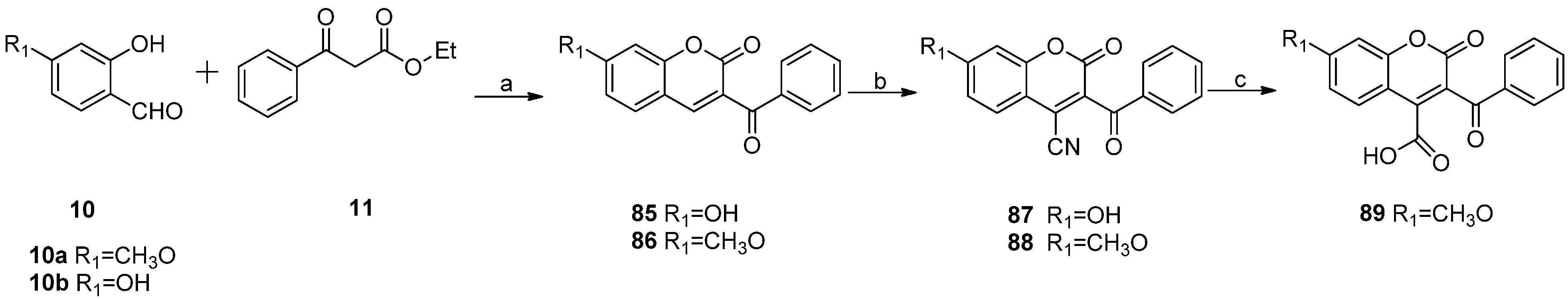
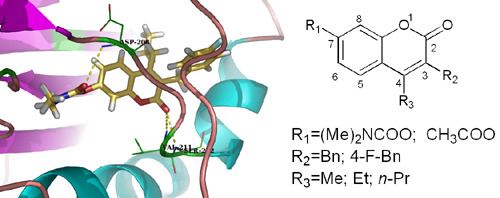
3.2.5. General Procedure for the Preparation of 7-Carboxylate-2H-chromen-2-one derivatives 13–82 [24]
To the solution of the 7-hydroxycoumarin derivatives 4 obtained above (1.0 equiv.) in DMF (5.5 mL/mmol) was added NaH (2.0 equiv.) at room temperature under a nitrogen atmosphere. The reaction mixture was stirred for 30 minutes and acylchloride (2.0 equiv.) was added. The reaction mixture was stirred for another hour and poured into saturated NaHCO3. After extraction with EtOAc three times the organic extract was combined washed with brine and dried over MgSO4. The products were purified by flash column chromatography.
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl dimethylcarbamate (13). Yield: 0.44 g, 86.8%, white solid, m.p.: 130–133 °C. 1H-NMR (CDCl3): δ 2.43 (s, 3H, CH3), 3.03 (s, 3H, NCH3), 3.12 (s, 3H, NCH3), 4.06 (s, 2H, CH2), 7.08–7.29 (m, 5H, PhH), 7.58 (d, J = 8.8 Hz, 1H, PhH). 13C-NMR (CDCl3): δ 15.48, 32.95, 36.56, 36.82, 110.10, 117.88, 118.22, 124.38, 125.15, 126.34, 128.26, 128.57, 138.82, 147.13, 152.91, 153.41, 153.99, 161.79. HRMS (ESI): m/z 338.13939 (M+H+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl dimethylcarbamate (14). Yield: 0.193 g, 52%, white solid, m.p.: 156–157 °C. 1H-NMR (CDCl3): δ 2.42 (s, 3H, CH3), 3.05 (s, 3H, NCH3), 3.17 (s, 3H, NCH3), 4.06 (s, 2H, CH2), 7.19–7.29 (m, 6H, PhH), 7.64 (s, 1H, PhH). 13C-NMR (CDCl3): δ 15.53, 33.04, 36.64, 36.98, 112.58, 119.13, 123.18, 125.36, 125.64, 126.47, 128.24, 128.64, 138.44, 146.02, 149.19, 151.16, 152.99, 161.22. HRMS (ESI): m/z 372.10060 (M+H+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl dimethylcarbamate (15). Yield: 0.147 g, 94.6%, white solid, m.p.: 85–86 °C. 1H-NMR (CDCl3): δ 1.14 (t, J = 7.6 Hz, 3H, CH3), 2.86 (q, J = 7.6 Hz, 2H, CH2CH3), 3.03 (s, 3H, NCH3), 3.12 (s, 3H, NCH3), 4.04 (s, 2H, CH2Ph), 7.08 (d, J = 2.4 Hz, 1H, H-8), 7.11–7.13 (dd, J = 2.4, 7.6 Hz, 1H, H-6), 7.17–7.27 (m, 5H, ArH), 7.60 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.20, 22.27, 32.54, 36.56, 36.82, 110.38, 116.66, 118.28, 123.42, 125.12, 126.33, 128.19, 128.56, 139.04, 152.68, 153.30, 153.48, 154.00, 162.11. HRMS (ESI): m/z 352.15437(M+H+), 374.13662 (M+Na+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl dimethylcarbamate (16). Yield: 0.44 g, 86.8%, white solid, m.p.: 117–118 °C. 1H-NMR (DMSO-d6): δ 0.97 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.38–1.45 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.84 (t, J = 8.0 Hz, 2H, CH2C2H5), 2.93 (s, 3H, NCH3), 3.06 (s, 3H, NCH3), 3.97 (s, 2H, CH2Ph), 7.15–7.27 (m, 7H, ArH), 7.83 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 14.46, 22.46, 31.15, 32.73, 36.56, 36.82, 110.33, 116.99, 118.21, 123.80, 125.22, 126.34, 128.18, 128.55, 139.08, 151.33, 153.27, 153.39, 153.99, 162.06. HRMS (ESI): m/z 366.17006 (M+H+), 388.15222 (M+Na+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl dimethylcarbamate (17). Yield: 0.173 g, 65.3%, white powder, m.p.: 149–150 °C. 1H-NMR (CDCl3): δ 2.44 (s, 3H, CH3), 3.03 (s, 3H, NCH3), 3.12 (s, 3H, NCH3), 4.02 (s, 2H, CH2), 6.93–6.97 (m, 2H, PhH), 7.09–7.11 (m, 2H, PhH), 7.20–7.23 (m, 2H, PhH), 7.59 (d, J = 8.6 Hz, 1H, PhH). 13C-NMR (CDCl3): δ 15.44, 32.20, 36.56, 36.82, 110.15, 115.24, 115.45, 117.78, 118.30, 124.25, 125.19, 129.65, 129.73, 134.42, 134.45, 147.11, 152.91, 153.50, 153.96, 160.32, 161.71, 162.74. HRMS (ESI): m/z 356.12960 (M+H+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl dimethylcarbamate (18). Yield: 0.090 g, yield 68%. M.p.: 75 °C. White solid. 1H-NMR (CDCl3): δ 1.14 (t, J = 7.6 Hz, 3H, CH3), 2.85 (q, J = 7.6 Hz, 2H, CH2CH3), 3.02 (s, 3H, NCH3), 3.12 (s, 3H, NCH3), 3.99 (s, 2H, CH2Ph), 6.94 (t, J = 8.0 Hz, 2H, PhH), 7.09–7.14 (m, 2H, H-6, H-8), 7.20–7.23 (m, J = 6.4 Hz, 2H, PhH), 7.60 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.28, 22.25, 31.77, 36.54, 36.79, 110.37, 115.17, 115.39, 116.51, 118.38, 123.16, 125.23, 129.60, 129.68, 134.72, 152.83, 153.40, 153.96, 160.27, 162.04, 162.70. HRMS (ESI): m/z 370.14511 (M+H+), 392.12716 (M+Na+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl dimethylcarbamate (19). Yield: 0.175 g, 91.6%, white solid, m.p.: 82–84 °C. 1H-NMR (CDCl3): δ 1.03 (t, J = 7.6 Hz, 3H, CH2CH2CH3), 1.52 (sext, J = 7.6 Hz, 2H, CH2CH2CH3), 2.80 (t, J = 7.6 Hz, 2H, CH2C2H5), 3.03 (s, 3H, NCH3), 3.12 (s, 3H, NCH3), 3.99 (s, 2H, CH2Ph), 6.92–6.97 (m, 2H, ArH), 7.08–7.13 (m, 2H, ArH), 7.19–7.23 (m, 2H, ArH), 7.58 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 14.45, 22.52, 31.12, 31.97, 36.55, 36.81, 110.35, 115.19, 115.40, 116.86, 118.32, 123.58, 125.31, 129.57, 129.65, 134.72, 151.43, 153.36, 153.96, 160.30, 161.99, 162.73. HRMS (ESI): m/z 384.16069 (M+H+), 406.14275 (M+Na+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl diethylcarbamate (20). Yield: 0.05 g, 27%, white solid, m.p.: 109 °C. 1H-NMR (CDCl3): δ 1.20–1.29 (dt, J = 6.8 Hz, 6H, CH2CH3), 2.44 (s, 3H, CH3), 3.39–3.46 (m, J = 6.8 Hz, 4H, CH2CH3), 4.06 (s, 2H, CH2Ph), 7.09–7.12 (m, 2H, ArH), 7.19–7.26 (m, 5H, ArH), 7.59 (d, J = 8.4 Hz, 1H, ArH). 13C-NMR (CDCl3): δ 13.33, 14.26, 15.50, 32.96, 42.01, 42.40, 110.05, 117.78, 118.22, 124.30, 125.12, 126.34, 128.27, 128.58, 138.84, 147.16, 152.93, 153.33, 153.48, 161.82. HRMS (ESI): m/z 366.16971 (M+H+), 388.15157 (M+Na+), 404.12558 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl diethylcarbamate (21). Yield: 0.069 g, 58%, white solid, m.p.: 83–84 °C. 1H-NMR (CDCl3): δ 1.21–1.32 (dt, J = 6.4 Hz, 6H, CH2CH3), 2.41 (s, 3H, CH3), 3.39–3.51 (m, J = 6.8 Hz, 4H, CH2CH3), 4.05 (s, 2H, CH2Ph), 7.18–7.27 (m, 6H, ArH), 7.64 (s, 1H, H-5). 13C-NMR (CDCl3): δ 13.27, 14.15, 15.53, 33.05, 42.21, 42.59, 112.54, 119.01, 123.30, 125.38, 125.55, 126.47, 128.25, 128.64, 138.48, 146.07, 149.28, 151.16, 152.34, 161.25. HRMS (ESI): m/z 400.13113 (M+H+), 422.11302 (M+Na+), 438.08702 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl diethylcarbamate (22). Yield: 0.118 g, 62.4%, milk white solid, m.p.: 64–65 °C. 1H-NMR (CDCl3): δ 1.15 (t, J = 7.6 Hz, 3H, CH3), 1.21–1.29 (dt, J = 6.8 Hz, 6H, N(CH2CH3)2), 2.87 (q, J = 7.6 Hz, 2H, CH2CH3), 3.38–3.49 (m, J = 6.8 Hz, 4H, N(CH2CH3)2), 4.05 (s, 2H, CH2Ph), 7.11–7.13 (m, 2H, H-6 and H-8), 7.20–7.30 (m, 5H, CH2Ph), 7.61 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.26, 13.35, 14.30, 22.30, 32.54, 42.06, 43.43, 110.33, 116.54, 118.34, 123.28, 125.17, 126.34, 128.20, 128.57, 139.08, 152.81, 153.37, 153.45, 162.17. HRMS (ESI): m/z 380.18545 (M+H+), 402.16736 (M+Na+), 418.14141 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl diethylcarbamate (23). Yield: 0.099 g, 50%, white solid, m.p.: 112–113 °C. 1H-NMR (CDCl3): δ 1.02 (3H, CH2CH2CH3), 1.21–1.23 (6H, CH2CH3), 1.50 (2H, CH2CH2CH3), 2.79 (2H, CH2CH2CH3), 3.41 (4H, CH2CH3), 4.03 (s, 2H, CH2Ph), 7.08–7.24 (m, 7H, ArH), 7.56 (d, J = 7.2 Hz, 1H, ArH). 13C-NMR (CDCl3): δ 18.30, 19.25, 19.42, 27.45, 36.11, 37.73, 47.04, 47.42, 115.22, 121.85, 123.17, 128.71, 130.20, 131.31, 133.18, 133.52, 144.13, 156.34, 158.30, 158.39, 167.03. HRMS (ESI): m/z 394.20179 (M+H+), 416.18395 (M+Na+), 432.15778 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl diethylcarbamate (24). Yield: 0.02 g, 10%, white solid, m.p.: 101–102 °C. 1H-NMR (CDCl3): δ 1.22–1.27 (m, 6H, CH2CH3), 2.44 (s, 3H, CH3), 3.39–3.45 (m, 4H, CH2CH3), 4.02 (s, 2H, CH2Ph), 6.95 (t, J = 8.0 Hz, 2H, ArH), 7.10–7.12 (m, 2H, H-6 and H-8), 7.22 (m, 2H, ArH), 7.60 (d, J = 8.0 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.33, 14.27, 15.48, 32.20, 42.03, 42.42, 110.09, 115.24, 115.45, 117.67, 118.32, 124.15, 125.18, 129.66, 129.74, 134.48, 147.18, 152.91, 153.30, 153.57, 160.30, 161.75, 162.73. HRMS (ESI): m/z 384.16108 (M+H+), 406.14280 (M+Na+), 422.11691 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl diethylcarbamate (25). Yield: 0.125 g, 63%, white solid, m.p.: 74–75 °C. 1H-NMR (CDCl3): δ 1.15 (t, J = 7.6 Hz, 3H, CH3), 1.20–1.29 (dt, J = 6.8 Hz, 6H, N(CH2CH3)2), 2.86 (q, J = 7.6 Hz, 2H, CH2CH3), 3.38–3.48 (m, J = 6.8 Hz, 4H, N(CH2CH3)2), 4.00 (s, 2H, CH2Ph), 6.96 (t, J = 8.4 Hz, 2H, ArH), 7.10 (d, 1H, H-6), 7.13 (s, 1H, H-8), 7.22 (m, 2H, ArH), 7.60 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 18.27, 19.24, 27.24, 36.79, 47.03, 47.42, 115.34, 120.20, 120.41, 121.42, 123.34, 128.16, 130.12, 134.58, 134.66, 139.67, 139.70, 157.73, 158.30, 158.47, 165.30, 167.03, 167.73. HRMS (ESI): m/z 398.17617 (M+H+), 420.15785 (M+Na+), 436.13206 (M+K+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl diethylcarbamate (26). Yield: 0.134 g, 65%, white solid, mp: 51–52 °C. 1H-NMR (CDCl3): δ 1.04 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.20–1.29 (m, 6H, CH2CH3), 1.52–1.53 (m, 2H, CH2CH2CH3), 2.80 (br s, 2H, CH2CH2CH3), 3.40–3.46 (m, 4H, CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.10–7.14 (m, 2H, H-6 and H-8), 7.20–7.23 (m, 2H, ArH), 7.58 (d, J = 8.4 Hz, 1H, H-5).13C-NMR (CDCl3): δ 13.35, 14.29, 14.50, 22.57, 31.15, 32.00, 42.09, 42.45, 110.31, 115.22, 115.44, 116.79, 118.35, 123.54, 125.32, 129.64, 134.79, 151.49, 153.35, 160.34, 162.04, 162.75. HRMS (ESI): m/z 412.19179 (M+H+), 434.17324 (M+Na+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl acetate (27). Yield: 0.05 g, 29%, white solid, m.p.: 190 °C. 1H-NMR (CDCl3): δ 2.39 (s, 3H, CH3CO), 2.43 (s, 3H, CH3), 4.06 (s, 2H, CH2Ph), 7.15 (s, 1H, H-8), 7.20–7.27 (m, 5H, CH2Ph), 7.68 (s, 1H, H-5). 13C-NMR (CDCl3): δ 15.56, 20.60, 33.07, 112.32, 119.73, 122.97, 125.69, 126.08, 126.53, 128.24, 128.67, 138.31, 145.88, 148.40, 151.16, 161.01, 167.94. HRMS (ESI): m/z 343.07294 (M+H+), 365.05485 (M+Na+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl acetate (28). Yield: 0.082 g, 55%, white solid, m.p.: 165–166 °C. 1H-NMR (CDCl3): δ 2.33 (s, 3H, CH3CO), 2.44 (s, 3H, CH3), 4.06 (s, 2H, CH2Ph), 7.05 (dd, J = 8.8, 2.0 Hz, 1H, H-6), 7.10 (d, J = 2.0 Hz, 1H, H-8), 7.18–7.29 (m, 5H, CH2Ph), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 15.51, 21.11, 32.97, 110.16, 118.00, 118.47, 124.85, 125.43, 126.40, 128.25, 128.60, 138.69, 146.98, 152.34, 152.90, 161.61, 168.82. HRMS (ESI): m/z 309.11175 (M+H+), 331.09374 (M+Na+), 347.06770 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl acetate (29). Yield: 0.11 g, 66%, white solid, m.p.: 123–125 °C. 1H-NMR (CDCl3): δ 1.14 (t, J = 7.6 Hz, 3H, CH2CH3), 2.34 (s, 3H, CH3CO), 2.86 (q, J = 7.6 Hz, 2H, CH2CH3), 4.04 (s, 2H, CH2Ph), 7.06 (d, J = 8.8 Hz, 1H, H-6), 7.12 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.20, 21.12, 22.28, 32.56, 110.43, 117.26, 118.05, 123.90, 125.39, 126.40, 128.19, 128.59, 138.91, 152.24, 152.52, 153.46, 161.91, 168.84. HRMS (ESI): m/z 323.12751 (M+H+), 345.10923 (M+Na+), 361.08309 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl acetate (30). Yield: 0.105 g, 63%, white solid, m.p.: 139–140 °C. 1H-NMR (CDCl3): δ 1.04 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.49–1.55 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.34 (s, 3H, CH3CO), 2.81 (t, J = 7.2 Hz, 2H, CH2CH2CH3), 4.04 (s, 2H, CH2Ph), 7.05 (d, J = 8.4 Hz, 1H, H-6), 7.11 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.60 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 14.48, 21.12, 22.46, 31.15, 32.75, 110.38, 117.58, 117.99, 124.28, 125.48, 126.40, 128.17, 128.58, 138.95, 151.17, 152.21, 153.38, 161.86, 168.83. HRMS (ESI): m/z 337.14307 (M+H+), 359.12512 (M+Na+), 375.09918 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl acetate (31). Yield: 0.100 g, 61%, white solid, m.p.: 119–120 °C. 1H-NMR (CDCl3): δ 2.34 (s, 3H, CH3CO), 2.44 (s, 3H, CH3), 4.02 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.06–7.11 (m, 2H, H-6 and H-8), 7.12 (s, 1H, H-8), 7.21–7.26 (m, 2H, ArH), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 15.47, 21.11, 32.21, 110.19, 115.26, 115.47, 118.09, 118.36, 124.69, 125.49, 129.66, 129.74, 134.31, 134.34, 146.97, 152.43, 152.88, 160.32, 161.52, 162.75, 168.80. HRMS (ESI): m/z 327.10315 (M+H+), 349.08500 (M+Na+), 365.05920 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl acetate (32). Yield: 0.12 g, 70%, white solid, m.p.: 122–123 °C. 1H-NMR (CDCl3): δ 1.16 (t, J = 7.6 Hz, 3H, CH2CH3), 2.34 (s, 3H, CH3CO), 2.86 (q, J = 7.6 Hz, 2H, CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.07 (d, J = 8.8 Hz, 1H, H-6), 7.12 (s, 1H, H-8), 7.20–7.26 (m, 2H, ArH), 7.63 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.27, 21.11, 22.26, 31.82, 110.47, 115.25, 115.46, 117.15, 118.14, 123.72, 125.43, 129.59, 129.67, 134.52, 134.55, 152.33, 152.56, 153.45, 160.33, 161.83, 162.76, 168.83. HRMS (ESI): m/z 341.11816 (M+H+), 363.10008 (M+Na+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl acetate (33). Yield: 0.127 g, 72%, white solid, m.p.: 150–151 °C. 1H-NMR (CDCl3): δ 1.07 (t, J = 7.6 Hz, 3H, CH2CH2CH3), 1.52–1.58 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.36 (s, 3H, CH3CO), 2.80–2.85 (m, 2H, CH2CH2CH3), 4.02 (s, 2H, CH2Ph), 6.98 (t, J = 8.4 Hz, 2H, ArH), 7.07–7.10 (dd, J = 2.0, 8.4 Hz, 1H, H-6), 7.13 (d, J = 2.0 Hz, 1H, H-8), 7.21–7.25 (m, 2H, ArH), 7.63 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 14.49, 21.12, 22.52, 31.14, 32.01, 110.43, 115.25, 115.46, 117.47, 118.08, 124.11, 125.54, 129.57, 129.65, 134.58, 151.20, 152.30, 153.37, 160.34, 161.78, 162.77, 168.82. HRMS (ESI): m/z 355.13436 (M+H+), 377.11631 (M+Na+), 393.09050 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl propionate (34). Yield: 0.047 g, 44%, white solid, m.p.: 124–126 °C. 1H-NMR (CDCl3): δ 1.32 (t, J = 7.2 Hz, 3H, CH2CH3), 2.43 (s, 3H, CH3), 2.69 (q, J = 7.2 Hz, 2H, CH2CH3), 4.06 (s, 2H, CH2Ph), 7.15 (s, 1H, H-8), 7.19–7.28 (m, 5H, CH2Ph), 7.67 (s, 1H, H-5). 13C-NMR (CDCl3): δ 8.99, 15.56, 27.45, 33.07, 112.33, 119.63, 123.00, 125.66, 126.01, 126.52, 128.25, 128.67, 138.34, 145.92, 148.51, 151.18, 161.05, 171.48. HRMS (ESI): m/z 357.08905 (M+H+), 379.07086 (M+Na+), 395.04497 (M+K+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl propionate (35). Yield: 0.14 g, 87%, white solid, m.p.: 118–119 °C. 1H-NMR (CDCl3): δ 1.28 (t, J = 7.6 Hz, 3H, CH2CH3), 2.45 (s, 3H, CH3), 2.63 (q, J = 7.6 Hz, 2H, CH2CH3), 4.07 (s, 2H, CH2Ph), 7.06 (d, J = 8.4 Hz, 1H, H-6), 7.10 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.61 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 8.93, 15.50, 27.74, 32.97, 110.12, 118.00, 118.38, 124.78, 125.38, 126.39, 128.26, 128.60, 138.71, 147.00, 152.50, 152.92, 161.64, 172.35. HRMS (ESI): m/z 323.12765 (M+H+), 345.10937 (M+Na+), 361.08330 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl propionate (36). Yield: 0.143 g, 85%, white solid, m.p.: 124 °C. 1H-NMR (CDCl3): δ 1.15 (t, J = 7.6 Hz, 3H, CH2CH3), 1.28 (t, J = 7.6 Hz, 3H, COCH2CH3), 2.63 (q, J = 7.6 Hz, 2H, CH2CH3), 2.87 (q, J = 7.6 Hz, 2H, COCH2CH3), 4.05 (s, 2H, CH2Ph), 7.06 (d, J = 8.8 Hz, 1H, H-6), 7.12 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 8.94, 13.21, 22.28, 27.74, 32.56, 110.39, 117.16, 118.06, 123.82, 125.34, 126.39, 128.20, 128.58, 138.93, 152.40, 152.54, 153.48, 161.95, 172.37. HRMS (ESI): m/z 337.14303 (M+H+), 359.12504 (M+Na+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl propionate (37). Yield: 0.121 g, 69%, white solid, m.p.: 120–121 °C. 1H-NMR (CDCl3): δ 1.04 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.28 (t, J = 7.6 Hz, 3H, COCH2CH3), 1.49–1.57 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.63 (q, J = 7.6 Hz, 2H, COCH2CH3), 2.81 (t, J = 8.0 Hz, 2H, CH2CH2CH3), 4.04 (s, 2H, CH2Ph), 7.05 (d, J = 8.8 Hz, 1H, H-6), 7.11 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.59 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 8.94, 14.48, 22.47, 27.74, 31.16, 32.75, 110.34, 117.48, 117.99, 124.19, 125.44, 126.40, 128.18, 128.57, 138.97, 151.20, 152.37, 153.39, 161.90, 172.35. HRMS (ESI): m/z 351.15891 (M+H+), 373.14083 (M+Na+), 389.11452 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl propionate (38). Yield: 0.082 g, 48%, white solid, m.p.: 115–116 °C. 1H-NMR (CDCl3): δ 1.28 (t, J = 7.6 Hz, 3H, CH2CH3), 2.45 (s, 3H, CH3), 2.63 (q, J = 7.6 Hz, 2H, CH2CH3), 4.02 (s, 2H, CH2Ph), 6.95 (t, J = 8.0 Hz, 2H, ArH), 7.06–7.10 (m, 2H, H-6 and H-8), 7.20–7.26 (m, 2H, ArH), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 8.93, 15.47, 27.73, 32.22, 110.16, 115.26, 115.48, 118.09, 118.27, 124.63, 125.43, 129.67, 129.75, 134.33, 134.36, 146.98, 152.59, 152.91, 160.33, 161.56, 162.76, 172.33. HRMS (ESI): m/z 341.11829 (M+H+), 363.10044 (M+Na+), 379.07431 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl propionate (39). Yield: 0.14 g, 81%, white solid, m.p.: 97–98 °C. 1H-NMR (CDCl3): δ 1.16 (t, J = 7.6 Hz, 3H, CH2CH3), 1.28 (t, J = 7.6 Hz, 3H, COCH2CH3), 2.63 (q, J = 7.6 Hz, 2H, CH2CH3), 2.87 (q, J = 7.6 Hz, 2H, COCH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.07 (d, J = 8.8 Hz, 1H, H-6), 7.12 (s, 1H, H-8), 7.20–7.24 (m, 2H, ArH), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 8.93, 13.27, 22.26, 27.74, 31.82, 110.43, 115.25, 115.46, 117.04, 118.14, 123.65, 125.38, 129.60, 129.68, 134.54, 134.57, 152.49, 152.57, 153.47, 160.33, 161.87, 162.76, 172.35. HRMS (ESI): m/z 355.13383 (M+H+), 377.11579 (M+Na+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl propionate (40). Yield: 0.113 g, 61%, white solid, m.p.: 94–95 °C. 1H-NMR (CDCl3): δ 1.05 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.28 (t, J = 7.6 Hz, 3H, COCH2CH3), 1.50–1.56 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.63 (q, J = 7.6 Hz, 2H, COCH2CH3), 2.81 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.06 (d, J = 8.8 Hz, 1H, H-6), 7.11 (s, 1H, H-8), 7.19–7.23 (m, 2H, ArH), 7.60 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 8.93, 14.49, 22.53, 27.74, 31.14, 32.01, 110.39, 115.24, 115.45, 117.37, 118.09, 124.03, 125.49, 129.58, 129.66, 134.58, 134.61, 151.23, 152.46, 153.38, 160.34, 161.82, 162.77, 172.35. HRMS (ESI): m/z 369.15020 (M+H+), 391.13211 (M+Na+), 407.10587 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl butyrate (41). Yield: 0.076 g, 68.5%, white solid, m.p.: 107–108 °C. 1H-NMR (CDCl3): δ 1.07 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.83 (m, 2H, CH2CH2CH3), 2.42 (s, 2H, CH3), 2.63 (t, J = 7.2 Hz, 2H, CH2CH2CH3), 4.05 (s, 2H, CH2Ph), 7.13 (s, 1H, H-8), 7.18–7.28 (m, 5H, ArH), 7.66 (s, 1H, H-5). 13C-NMR (CDCl3): δ13.65, 15.55, 18.34, 33.07, 35.84, 112.34, 119.62, 123.00, 125.67, 125.99, 126.52, 128.25, 128.66, 138.35, 145.93, 148.50, 151.16, 161.03, 170.64. HRMS (ESI): m/z 371.10463 (M+H+), 393.08669 (M+Na+), 409.06073 (M+K+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl butyrate (42). Yield: 0.15 g, 89%, white solid, m.p.: 139–140 °C. 1H-NMR (CDCl3): δ 1.05 (t, J = 7.6 Hz, 3H, CH2CH3), 1.80 (q, J = 7.6 Hz, 2H, CH2CH3), 2.44 (s, 3H, CH3), 2.57 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 4.06 (s, 2H, CH2Ph), 7.05 (d, J = 8.8 Hz, 1H, H-6), 7.09 (s, 1H, H-8), 7.19–7.26 (m, 5H, CH2Ph), 7.61 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.61, 15.51, 18.34, 32.97, 36.17, 110.14, 118.04, 118.37, 124.77, 125.39, 126.39, 128.26, 128.60, 138.72, 147.00, 152.48, 152.91, 161.63, 171.52. HRMS (ESI): m/z 337.14308 (M+H+), 359.12508 (M+Na+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl butyrateen-7-yl propionate (43). Yield: 0.18 g, 100%, white solid, m.p.: 102 °C. 1H-NMR (CDCl3): δ 1.06 (t, J = 7.2 Hz, 3H, COCH2CH2CH3), 1.14 (t, J = 7.6 Hz, 3H, CH2CH3), 1.78–1.83 (m, J = 7.2 Hz, 2H, COCH2CH2CH3), 2.58 (t, J = 7.2 Hz, 2H, COCH2CH2CH3), 2.87 (q, J = 7.6 Hz, 2H, CH2CH3), 4.04 (s, 2H, CH2Ph), 7.05 (d, J = 8.8 Hz, 1H, H-6), 7.11 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.20, 13.61, 22.28, 29.71, 32.57, 36.17, 110.42, 117.15, 118.08, 123.82, 125.34, 126.38, 128.19, 128.58, 138.94, 152.38, 152.53, 153.48, 161.93, 171.55. HRMS (ESI): m/z 351.15891 (M+H+), 373.14081 (M+Na+), 389.11448 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl butyrate (44). Yield: 0.167 g, 92%, white solid, m.p.: 105–106 °C. 1H-NMR (CDCl3): δ 1.02–1.07 (m, J = 7.6 Hz, 6H, CH3), 1.49–1.55 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 1.77–1.83 (m, J = 7.6 Hz, 2H, COCH2CH2CH3), 2.58 (t, J = 7.2 Hz, 2H, COCH2CH2CH3), 2.81 (t, J =8.0 Hz, 2H, CH2CH2CH3), 4.04 (s, 2H, CH2Ph), 7.04 (d, J = 8.8 Hz, 1H, H-6), 7.10 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2Ph), 7.59 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.60, 14.48, 18.35, 22.47, 31.15, 32.76, 36.17, 110.37, 117.48, 118.03, 124.19, 125.45, 126.40, 128.18, 128.57, 138.98, 151.20, 152.36, 153.39, 161.89, 171.54. HRMS (ESI): m/z 365.17454 (M+H+), 387.15649 (M+Na+), 403.13042 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl butyrate (45). Yield: 0.122 g, 69%, white solid, m.p.: 85–86 °C. 1H-NMR (CDCl3): δ 1.05 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.77–1.83 (m, J = 7.2 Hz, 2H, CH2CH2CH3), 2.45 (s, 3H, CH3), 2.58 (t, J = 7.2Hz, 2H, CH2CH2CH3), 4.02 (s, 2H, CH2Ph), 6.95 (t, J = 8.0 Hz, 2H, ArH), 7.05–7.10 (m, 2H, H-6 and H-8), 7.20–7.26 (m, 2H, ArH), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.61, 15.47, 18.34, 32.22, 36.16, 110.18, 115.26, 115.47, 118.13, 118.27, 124.63, 125.43, 129.67, 129.75, 134.33, 134.36, 146.99, 152.57, 152.90, 160.32, 161.54, 162.75, 171.51. HRMS (ESI): m/z 355.13422 (M+H+), 377.11617 (M+Na+), 393.09024 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl butyrate (46). Yield: 0.155 g, 84%, white solid, m.p.: 79 °C. 1H-NMR (CDCl3): δ 1.06 (t, J = 7.6 Hz, 3H, CH2CH2CH3), 1.16 (t, J = 7.6 Hz, 3H, CH2CH3), 1.78–1.83 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.58 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 2.87 (q, J = 7.6 Hz, 2H, CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.06 (d, J = 8.4 Hz, 1H, H-6), 7.11 (s, 1H, H-8), 7.20–7.24 (m, 2H, ArH), 7.62 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.27, 13.60, 18.34, 22.26, 31.82, 36.17, 110.46, 115.25, 115.46, 117.04, 118.17, 123.66, 125.37, 129.59, 129.67, 134.53, 134.57, 152.47, 152.56, 153.47, 160.34, 161.85, 162.77, 171.54. HRMS (ESI): m/z 369.14937 (M+H+), 391.13124 (M+Na+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl butyrate (47). Yield: 0.122 g, 64%, white solid, m.p.: 88–90 °C. 1H-NMR (CDCl3): δ 1.05–1.07 (m, 6H, CH3), 1.50–1.56 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 1.77–1.83 (m, J = 7.2 Hz, 2H, COCH2CH2CH3), 2.58 (t, J = 7.2 Hz, 2H, COCH2CH2CH3), 2.80 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.05 (d, J = 8.8 Hz, 1H, H-6), 7.10 (s, 1H, H-8), 7.21–7.22 (m, 2H, ArH), 7.60 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.60, 14.49, 18.35, 22.53, 31.14, 32.01, 36.17, 110.42, 115.24, 115.45, 117.37, 118.12, 124.03, 125.48, 129.58, 129.65, 134.58, 151.22, 152.44, 153.38, 160.34, 161.81, 162.77, 171.53. HRMS (ESI): m/z 383.16575 (M+H+), 405.14765 (M+Na+), 421.12162 (M+K+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl pentanoate (48). Yield: 0.151 g, 86%, white solid, m.p.: 110–111 °C. 1H-NMR (CDCl3): δ 0.98 (t, J = 7.6 Hz, 3H, CH2CH3), 1.43–1.48 (m, J = 7.6 Hz, 2H, CH2CH3), 1.73–1.77 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.44 (s, 3H, CH3), 2.59 (t, J = 7.6 Hz, 2H, COCH2), 4.06 (s, 2H, CH2Ph), 7.05 (d, J = 8.8 Hz, 1H, H-6), 7.09 (s, 1H, H-8), 7.18–7.25 (m, 5H, CH2Ph), 7.61 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.71, 15.50, 22.23, 26.86, 32.97, 34.07, 110.13, 118.03, 118.37, 124.76, 125.39, 126.38, 128.27, 128.60, 138.73, 147.00, 152.50, 152.91, 161.62, 171.70. HRMS (ESI): m/z 351.15892 (M+H+), 373.14081 (M+Na+), 389.11455 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl pentanoate (49). Yield: 0.099 g, 86%, white solid, m.p.: 108–109 °C. 1H-NMR (CDCl3): δ 0.98 (t, J = 7.2 Hz, 3H, CH2CH3), 1.45–1.50 (m, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 1.74–1.80 (m, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 2.42 (s, 3H, CH3), 2.65 (t, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 4.05 (s, 2H, CH2Ph), 7.13 (s, 1H, H-8), 7.19–7.29 (m, 5H, CH2PhH), 7.66 (s, 1H, H-5). 13C-NMR (CDCl3): δ 13.70, 15.55, 22.23, 26.82, 33.07, 33.72, 112.34, 119.62, 123.00, 125.67, 125.99, 126.52, 128.25, 128.66, 138.35, 145.93, 148.51, 151.16, 161.03, 170.81. HRMS (ESI): m/z 385.12011 (M+H+), 407.10215 (M+Na+), 423.07623 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl pentanoate (50). Yield: 0.112 g, 62%, white solid, m.p.: 72–73 °C. 1H-NMR (CDCl3): δ 0.98 (t, J = 7.2 Hz, 3H, CH2CH2CH2CH3), 1.14 (t, J = 6.8 Hz, 3H, CH2CH3), 1.43–1.48 (m, J = 6.8 Hz, 2H, CH2CH2CH2CH3), 1.74–1.77 (m, J = 6.8 Hz, 2H, CH2CH2CH2CH3), 2.59 (t, J = 6.8 Hz, 2H, COCH2), 2.86–2.87 (m, J = 6.0 Hz, 2H, CH2CH3), 4.04 (s, 2H, CH2Ph), 7.04–7.10 (m, 2H, H-6 and H-8), 7.18–7.25 (m, 5H, CH2PhH), 7.61 (d, J = 6.0 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 18.17, 18.70, 27.21, 27.26, 31.85, 37.56, 39.06, 115.40, 122.14, 123.06, 128.81, 130.32, 131.37, 133.18, 133.57, 143.93, 157.39, 157.51, 158.47, 166.92, 176.71. HRMS (ESI): m/z 365.17447 (M+H+), 387.15629 (M+Na+), 403.13036 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl pentanoate (51). Yield: 0.166 g, 88%, white solid, m.p.: 78–79 °C. 1H-NMR (CDCl3): δ 0.95–1.05 (m, 6H, CH3), 1.43–1.53 (m, 4H, CH2CH2CH2CH3 and CH2CH2CH3), 1.74–1.79 (m, 2H, CH2CH2CH2CH3), 2.57–2.61 (m, 2H, CH2CH2CH3), 2.78–2.82 (m, 2H, COCH2), 4.04 (s, 2H, CH2Ph), 7.03–7.09 (m, 2H, H-6 and H-8), 7.18–7.25 (m, 5H, CH2PhH), 7.57–7.59 (m, 1H, H-5). 13C-NMR (CDCl3): δ 13.71, 14.48, 22.22, 22.47, 26.86, 31.15, 32.75, 34.07, 110.36, 117.47, 118.03, 124.19, 125.44, 126.40, 128.18, 128.57, 138.98, 151.20, 152.37, 153.39, 161.89, 171.73. HRMS (ESI): m/z 379.19074 (M+H+), 401.17263 (M+Na+), 417.14655 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl pentanoate (52). Yield: 0.153 g, 80%, white solid, m.p.: 70–71 °C. 1H-NMR (CDCl3): δ 0.98 (t, J = 7.6 Hz, 3H, CH2CH2CH2CH3), 1.16 (t, J = 7.6 Hz, 3H, CH2CH3), 1.43–1.49 (m, J = 7.6 Hz, 2H, CH2CH2CH2CH3), 1.72–1.80 (m, J = 7.6 Hz, 2H, CH2CH2CH2CH3), 2.60 (t, J = 7.6 Hz, 2H, COCH2), 2.87 (q, J = 7.6 Hz, 2H, CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.06 (d, J = 8.8 Hz, 1H, H-6), 7.11 (s, 1H, H-8), 7.20–7.23 (m, 2H, ArH), 7.62 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.27, 13.70, 22.22, 22.26, 26.86, 31.82, 34.06, 110.46, 115.25, 115.46, 117.04, 118.17, 123.65, 125.37, 129.60, 129.67, 134.54, 134.57, 152.49, 152.57, 153.47, 160.34, 161.85, 162.76, 171.72. HRMS (ESI): m/z 383.16509 (M+H+), 405.14697 (M+Na+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl pentanoate (53). Yield: 0.122 g, 66%, white solid, m.p.: 83–84 °C. 1H-NMR (CDCl3): δ 0.98 (t, J = 7.2 Hz, 3H, CH2CH3), 1.43–1.48 (m, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 1.72–1.79 (m, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 2.44 (s, 3H, CH3), 2.59 (t, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 4.02 (s, 2H, CH2Ph), 6.95 (t, J = 8.0 Hz, 2H, ArH), 7.05–7.09 (m, 2H, H-6 and H-8), 7.20–7.26 (m, 2H, ArH), 7.62 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.70, 15.47, 22.22, 26.85, 32.22, 34.06, 110.17, 115.26, 115.47, 118.12, 118.26, 124.62, 125.43, 129.67, 129.75, 134.33, 134.36, 146.98, 152.59, 152.90, 160.32, 161.54, 162.75, 171.69. HRMS (ESI): m/z 369.15012 (M+H+), 391.13209 (M+Na+), 407.10611 (M+K+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl pentanoate (54). Yield: 0.122 g, 62%, white solid, m.p.: 68–70 °C. 1H-NMR (CDCl3): δ 0.98 (t, J = 7.2 Hz, 3H, CH2CH2CH2CH3), 1.05 (t, J = 7.2 Hz, 3H, CH2CH2CH3), 1.43–1.56 (m, 4H, CH2CH2CH2CH3 and CH2CH2CH3), 1.72–1.79 (m, J = 7.2 Hz, 2H, CH2CH2CH2CH3), 2.60 (t, J = 7.2 Hz, 2H, COCH2), 2.78–2.82 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 4.00 (s, 2H, CH2Ph), 6.94–6.98 (m, 2H, CH2PhH), 7.05 (d, J = 8.8Hz, 1H, H-6), 7.10 (s, 1H, H-8), 7.20–7.23 (m, 2H, CH2PhH), 7.60 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.70, 14.48, 22.22, 22.53, 26.86, 31.14, 32.01, 34.07, 110.41, 115.24, 115.45, 117.36, 118.11, 124.02, 125.48, 129.58, 129.66, 134.58, 134.61, 151.22, 152.46, 153.38, 160.34, 161.81, 162.77, 171.71. HRMS (ESI): m/z 397.18122 (M+H+), 419.16309 (M+Na+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl pivalate (55). Yield: 0.157 g, 90%, white solid, m.p.: 157–158 °C. 1H-NMR (CDCl3): δ 1.37 (s, 9H, C(CH3)3), 2.44 (s, 3H, CH3), 4.06 (s, 2H, CH2Ph), 7.01 (d, J = 8.8 Hz, 1H, H-6), 7.06 (s, 1H, H-8), 7.19–7.26 (m, 5H, CH2Ph), 7.61 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 15.51, 27.08, 32.99, 39.24, 110.06, 117.98, 118.29, 124.72, 125.33, 126.38, 128.28, 128.60, 138.75, 147.00, 152.94, 161.63, 176.52. HRMS (ESI): m/z 351.15890 (M+H+), 373.14087 (M+Na+), 389.11450 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl pivalate (56). Yield: 0.1 g, 87%, white solid, m.p.: 113 °C. 1H-NMR (CDCl3): δ 1.41 (s, 9H, C(CH3)3), 2.43 (s, 3H, CH3), 4.06 (s, 2H, CH2Ph), 7.11 (s, 1H, H-8), 7.20–7.28 (m, 5H, CH2PhH), 7.66 (s, 1H, H-5). 13C-NMR (CDCl3): δ 15.55, 27.14, 33.08, 39.43, 112.26, 119.52, 123.06, 125.64, 125.94, 126.51, 128.27, 128.66, 138.38, 145.92, 148.81, 151.19, 161.06, 175.56. HRMS (ESI): m/z 385.12023 (M+H+), 407.10224 (M+Na+), 423.07644 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl pivalate (57). Yield: 0.180 g, 99%, white solid, m.p.: 105–106 °C. 1H-NMR (CDCl3): δ 1.15 (t, J = 7.6 Hz, 3H, CH2CH3), 1.38 (s, 9H, C(CH3)3), 2.87 (q, J = 7.6 Hz, 2H, CH2CH3), 4.05 (s, 2H, CH2Ph), 7.01–7.03 (dd, J = 1.6, 8.4 Hz, 1H, H-6), 7.07 (d, J = 2.0 Hz, 1H, H-8), 7.19–7.30 (m, 5H, CH2Ph), 7.61 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.23, 22.29, 27.08, 32.57, 39.25, 110.34, 117.06, 118.04, 123.76, 125.30, 126.39, 128.21, 128.59, 138.95, 152.57, 152.83, 153.50, 161.97, 176.58. HRMS (ESI): m/z 365.17453 (M+H+), 387.15631 (M+Na+), 403.13040 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl pivalate (58). Yield: 0.184 g, 97%, white solid, m.p.: 138–139 °C. 1H-NMR (CDCl3): δ 1.04 (t, J = 6.4 Hz, 3H, CH2CH3), 1.38 (s, 9H, C(CH3)3), 1.52 (q, J = 7.2 Hz, 2H, CH2CH2CH3), 2.81 (m, 2H, CH2CH2CH3), 4.04 (s, 2H, CH2Ph), 7.01 (d, J = 8.4 Hz, 1H, H-6), 7.07 (s, 1H, H-8), 7.19–7.26 (m, 5H, CH2Ph), 7.58 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 14.48, 22.48, 27.08, 31.15, 32.77, 39.25, 110.28, 117.39, 117.96, 124.14, 125.39, 126.39, 128.20, 128.57, 139.00, 151.21, 152.82, 153.42, 161.90, 176.54. HRMS (ESI): m/z 379.19072 (M+H+), 401.17258 (M+Na+), 417.14652 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl pivalate (59). Yield: 0.16 g, 84%, white solid, m.p.: 103–104 °C. 1H-NMR (CDCl3): δ 1.16 (t, J = 7.6 Hz, 3H, CH2CH3), 1.38 (s, 9H, C(CH3)3), 2.87 (q, J = 7.6 Hz, 2H, CH2CH3), 4.00 (s, 2H, CH2Ph), 6.95 (t, J = 8.4 Hz, 2H, ArH), 7.03 (d, J = 8.8 Hz, 1H, H-6), 7.08 (s, 1H, H-8), 7.21–7.26 (m, 2H, ArH), 7.62 (d, J = 8.4 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 13.28, 22.27, 27.07, 31.83, 39.25, 110.37, 115.24, 115.45, 116.95, 118.11, 123.59, 125.34, 129.62, 129.69, 134.57, 134.60, 152.58, 152.94, 153.50, 160.33, 161.86, 162.76, 176.53. HRMS (ESI): m/z 383.16508 (M+H+), 405.14694 (M+Na+), 421.11022 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl pivalate (60). Yield: 0.096 g, 52%, white solid, m.p.: 136–138 °C. 1H-NMR (CDCl3): δ 1.38 (s, 9H, C(CH3)3), 2.45 (s, 3H, CH3), 4.02 (s, 2H, CH2Ph), 6.96 (t, J = 8.0 Hz, 2H, ArH), 7.01–7.06 (m, 2H, H-6 and H-8), 7.21–7.26 (m, 2H, ArH), 7.60 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 15.48, 27.07, 32.24, 39.25, 110.11, 115.27, 115.48, 118.06, 118.19, 124.59, 125.37, 129.68, 129.76, 134.35, 134.38, 146.98, 152.94, 153.04, 160.33, 161.55, 162.76, 176.52. HRMS (ESI): m/z 369.15012 (M+H+), 391.13212 (M+Na+), 407.10589 (M+K+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl pivalate (61). Yield: 0.160 g, 81%, white solid, m.p.: 138–140 °C. 1H-NMR (CDCl3): δ 1.05 (t, J = 7.2 Hz, 3H, CH2CH3), 1.38 (s, 9H, C(CH3)3), 1.52–1.56 (m, J = 7.2 Hz, 2H, CH2CH2CH3), 2.81 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 4.00 (s, 2H, CH2Ph), 6.96 (t, J = 8.4 Hz, 2H, ArH), 7.02 (d, J =8.8 Hz, 1H, H-6), 7.07 (s, 1H, H-8), 7.20–7.23 (m, 2H, ArH), 7.59 (d, J = 8.8 Hz, 1H, H-5). 13C-NMR (CDCl3): δ 14.48, 22.53, 27.07, 31.14, 32.02, 39.25, 110.33, 115.24, 115.45, 117.28, 118.05, 123.98, 125.42, 129.59, 129.67, 134.60, 134.63, 151.22, 152.91, 153.41, 160.34, 161.82, 162.77, 176.53. HRMS (ESI): m/z 397.18136 (M+H+), 419.16318 (M+Na+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl benzoate (62). Yield: 0.180 g, 97%, white solid, m.p.: 149–150 °C. 1H-NMR (CDCl3): δ 2.47 (s, 3H, CH3), 4.08 (s, 2H, CH2Ph), 7.18–7.27 (m, 7H, ArH), 7.53 (t, 2H, ArH), 7.67 (t, 2H, ArH), 8.21 (d, J = 7.6 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 15.55, 33.01, 110.33, 118.18, 118.55, 124.88, 125.49, 126.41, 128.29, 128.62, 128.72, 128.91, 130.33, 134.02, 138.72, 147.03, 152.69, 152.99, 161.64, 164.62. HRMS (ESI): m/z 371.12762 (M+H+), 393.10954 (M+Na+), 409.08341 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl benzoate (63). Yield: 0.179 g, 88.6%, white solid, m.p.: 133–135 °C. 1H-NMR (CDCl3): δ 2.46 (s, 3H, CH3), 4.08 (s, 2H, CH2Ph), 7.21–7.32 (m, 6H, ArH), 7.53–7.57 (m, 2H, ArH), 7.67–7.72 (m, 2H, ArH), 8.23–8.25 (m, J = 7.2 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 15.59, 33.10, 112.47, 119.71, 123.11, 125.71, 126.08, 126.54, 128.27, 128.68, 128.78, 130.53, 134.26, 138.36, 145.94, 148.67, 151.21, 161.08, 163.77. HRMS (ESI): m/z 405.08872 (M+H+), 427.07096 (M+Na+), 443.04450 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl benzoate (64). Yield: 0.149 g, 78%, white solid, m.p.: 138 °C. 1H-NMR (CDCl3): δ 1.17 (t, J = 6.8 Hz, 3H, CH2CH3), 2.88–2.90 (m, J = 7.2 Hz, 2H, CH2CH3), 4.06 (s, 2H, CH2Ph), 7.18–7.27 (m, 7H, ArH), 7.52–7.55 (m, 2H, ArH), 7.65–7.69 (m, 2H, ArH), 8.21 (d, J = 6.4 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 18.20, 27.29, 37.60, 115.58, 122.32, 123.18, 128.94, 130.41, 131.38, 133.21, 133.58, 133.69, 133.94, 135.31, 138.99, 143.94, 157.50, 157.60, 158.57, 166.90, 169.60. HRMS (ESI): m/z 385.14322 (M+H+), 407.12515 (M+Na+), 423.09884 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl benzoate (65). Yield: 0.159 g, 80.3%, white solid, m.p.: 120 °C. 1H-NMR (CDCl3): δ 1.05 (t, J = 7.2 Hz, 3H, CH2CH3), 1.52–1.57 (m, J = 7.2 Hz, 2H, CH2CH3), 2.83 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 4.06 (s, 2H, CH2Ph), 7.18–7.27 (m, 7H, ArH), 7.48–7.55 (m, 2H, ArH), 7.64–7.68 (m, 2H, ArH), 8.21 (d, J = 7.2 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 14.51, 22.50, 31.19, 32.79, 110.56, 117.66, 118.18, 124.30, 125.56, 126.42, 128.21, 128.60, 128.72, 130.34, 134.03, 138.98, 151.24, 152.57, 153.46, 161.92, 164.64. HRMS (ESI): m/z 399.15917 (M+H+), 421.14128 (M+Na+), 437.11514 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl benzoate (66). Yield: 0.13 g, 67%, white solid, m.p.: 151–153 °C. 1H-NMR (CDCl3): δ 2.48 (s, 3H, CH3), 4.04 (s, 2H, CH2Ph), 6.97 (t, J = 8.4 Hz, 2H, ArH), 7.20–7.26 (m, 4H, ArH), 7.52–7.56 (m, 2H, ArH), 7.65–7.69 (m, 2H, ArH), 8.21 (d, J = 7.6 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 15.51, 32.25, 110.39, 115.29, 115.50, 118.26, 118.44, 124.75, 125.53, 128.72, 128.87, 129.69, 129.77, 130.33, 134.04, 134.35, 146.99, 152.79, 152.99, 160.35, 161.56, 162.78,164.61. HRMS (ESI): m/z 389.11849 (M+H+), 411.10049 (M+Na+), 427.07456 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl benzoate (67). Yield: 0.073 g, 36%, white solid, m.p.: 70–71 °C. 1H-NMR (CDCl3): δ 1.18 (t, J = 7.2 Hz, 3H, CH2CH3), 2.89 (q, J = 7.2 Hz, 2H, CH2CH3), 4.02 (s, 2H, CH2Ph), 6.96 (t, J = 8.0 Hz, 2H, ArH), 7.22–7.25 (m, 4H, ArH), 7.53–7.69 (m, 4H, ArH), 8.21 (d, J = 7.2 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 18.27, 27.27, 36.85, 115.62, 120.24, 120.46, 122.21, 123.27, 128.77, 130.45, 133.70, 133.90, 134.61, 134.69, 135.31, 139.01, 139.54, 157.53, 157.70, 158.55, 166.82, 167.78, 169.59. HRMS (ESI): m/z 403.13421 (M+H+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl benzoate (68). Yield: 0.176 g, 85%, white solid, m.p.: 95–96 °C. 1H-NMR (CDCl3): δ 1.06 (t, J = 7.2 Hz, 3H, CH2CH3), 1.53–1.58 (m, J = 7.2 Hz, 2H, CH2CH2CH3), 2.83 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 4.02 (s, 2H, CH2Ph), 6.96 (t, J = 8.4 Hz, 2H, ArH), 7.19–7.25 (m, 4H, ArH), 7.52–7.55 (m, 2H, ArH), 7.65–7.69 (m, 2H, ArH), 8.21 (d, J = 8.0 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 14.51, 22.56, 31.18, 32.04, 110.60, 115.26, 115.47, 117.54, 118.26, 124.13, 125.60, 128.73, 128.87, 129.61, 129.68, 130.33, 134.05, 134.59, 134.62, 151.26, 152.66, 153.45, 160.35, 161.83, 162.78, 164.62. HRMS (ESI): m/z 417.14989 (M+H+), 439.13191 (M+Na+), 455.10528 (M+K+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate (69). Yield: 0.138 g, 68%, white solid, m.p.: 131–132 °C. 1H-NMR (CDCl3): δ 2.43 (s, 3H, CH3), 4.04 (s, 2H, CH2Ph), 6.84 (s, 1H, H-8), 7.06 (d, J = 8.8 Hz, 1H, H-6), 7.19–7.29 (m, 5H, CH2Ph), 7.53–7.58 (m, 3H, H-5 and PhSO3), 7.70 (t, J = 7.6 Hz, 1H, PhSO3), 7.86 (d, J = 7.6 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 15.54, 33.03, 110.61, 118.69, 119.57, 125.67, 125.80, 126.51, 128.31, 128.46, 128.64, 129.43, 134.70, 135.03, 138.45, 146.56, 150.76, 152.59, 161.20. HRMS (ESI): m/z 407.09446 (M+H+), 429.07635 (M+Na+), 445.05041 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate (70). Yield: 0.165 g, 75%, white solid, m.p.: 154–155 °C. 1H-NMR (CDCl3): δ 2.41 (s, 3H, CH3), 4.05 (s, 2H, CH2Ph), 7.19–7.25 (m, 6H, ArH), 7.58–7.60 (m, 3H, ArH), 7.73 (m, 1H, ArH), 7.93 (d, J = 6.4 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 15.54, 33.13, 112.33, 120.38, 123.50, 126.11, 126.62, 126.78, 128.30, 128.60, 128.70, 129.47, 134.91, 135.37, 138.15, 145.50, 146.48, 150.83, 160.73. HRMS (ESI): m/z 441.05606 (M+H+), 463.03794 (M+Na+), 479.01170 (M+K+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl benzenesulfonate (71). Yield: 0.187 g, 89%, white solid, m.p.: 57–58 °C. 1H-NMR (CDCl3): δ 1.11 (t, J = 7.2 Hz, 3H, CH2CH3), 2.84 (t, J = 7.2 Hz, 2H, CH2CH3), 4.01 (s, 2H, CH2Ph), 6.88 (s, 1H, H-8), 7.03 (d, J = 8.8 Hz, 1H, H-6), 7.16–7.24 (m, 5H, CH2Ph), 7.52–7.58 (m, 3H, H-5 and PhSO3), 7.67–7.70 (m, J = 7.2 Hz, 1H, PhSO3), 7.86 (d, J = 8.0 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 18.15, 27.28, 37.62, 115.83, 123.38, 123.66, 129.67, 130.88, 131.48, 133.24, 133.42, 133.61, 134.48, 139.76, 140.02, 143.69, 155.63, 157.23, 158.14, 166.51. HRMS (ESI): m/z 421.11015 (M+H+), 443.09190 (M+Na+), 459.06604 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl benzenesulfonate (72). Yield: 0.155 g, 71%, white solid, m.p.: 96 °C. 1H-NMR (CDCl3): δ 1.03 (br s, 3H, CH2CH3), 1.49 (br s, 2H, CH2CH3), 2.79 (br s, 2H, CH2CH2CH3), 4.02 (s, 2H, CH2Ph), 6.84 (s, 1H, H-8), 7.06 (d, J = 6.8 Hz, 1H, H-6), 7.24 (m, 5H, CH2Ph), 7.56 (br s, 3H, H-5 and PhSO3), 7.71 (br s, 1H, PhSO3), 7.87 (d, J = 6.0 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 14.48, 22.43, 31.15, 32.81, 110.81, 118.71, 125.07, 125.86, 126.52, 128.21, 128.46, 128.62, 129.45, 134.71, 135.09, 138.69, 150.64, 150.80, 153.06, 161.48. HRMS (ESI): m/z 435.12627 (M+H+), 457.10839 (M+Na+), 473.08227 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl benzenesulfonate (73). Yield: 0.195 g, 89%, white solid, m.p.: 93–94 °C. 1H-NMR (CDCl3): δ 1.14 (t, J = 7.6 Hz, 3H, CH2CH3), 2.85 (q, J = 7.6 Hz, 2H, CH2CH3), 3.97 (s, 2H, CH2Ph), 6.87 (s, 1H, H-8), 6.94 (t, J = 8.4 Hz, 2H, CH2Ph), 7.07 (d, J = 8.4 Hz, 1H, H-6), 7.19–7.23 (m, 2H, CH2Ph), 7.55–7.60 (m, 3H, H-5 and PhSO3), 7.69–7.73 (m, 1H, PhSO3), 7.87 (d, J = 8.0 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 13.22, 22.26, 31.87, 110.89, 115.28, 115.49, 118.26, 118.79, 124.53, 125.83, 128.44, 129.45, 129.65, 129.73, 134.27, 134.30, 134.74, 135.08, 150.73, 152.19, 153.15, 160.36, 161.44, 162.79. HRMS (ESI): m/z 439.10053 (M+H+), 461.08240 (M+Na+), 477.05639 (M+K+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate (74). Yield: 0.153 g, 72%, white solid, m.p.: 108–109 °C. 1H-NMR (CDCl3): δ 2.44 (s, 3H, CH3), 3.99 (s, 2H, CH2Ph), 6.85 (s, 1H, H-8), 6.93–6.97 (m, J = 8.4 Hz, 2H, CH2Ph), 7.07 (d, J = 8.8 Hz, 1H, H-6), 7.19–7.23 (m, 2H, CH2Ph), 7.54–7.59 (m, 3H, H-5 and PhSO3), 7.69–7.71 (m, 1H, PhSO3), 7.86 (d, J = 7.6 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 15.50, 32.27, 110.65, 115.31, 115.52, 118.76, 119.47, 125.52, 125.86, 128.45, 129.44, 129.72, 129.80, 134.05, 134.09, 134.72, 135.03, 146.56, 150.84, 152.59, 160.36, 161.13, 162.80. HRMS (ESI): m/z 425.08559 (M+H+), 447.06760 (M+Na+), 463.04156 (M+K+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl benzenesulfonate (75). Yield: 0.104 g, 46%, white solid, m.p.: 109–110 °C. 1H-NMR (CDCl3): δ 1.04 (t, J = 7.2 Hz, 3H, CH2CH3), 1.48–1.54 (m, J = 7.6 Hz, 2H, CH2CH2CH3), 2.79 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 3.97 (s, 2H, CH2Ph), 6.86 (s, 1H, H-8), 6.95 (t, J = 8.4 Hz, 2H, CH2Ph), 7.07 (d, J = 8.8 Hz, 1H, H-6), 7.19–7.22 (m, 2H, CH2Ph), 7.55–7.59 (m, 3H, H-5 and PhSO3), 7.69–7.73 (m, 1H, PhSO3), 7.88 (d, J = 7.6 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 14.48, 22.48, 31.13, 32.05, 110.85, 115.29, 115.50, 118.56, 118.76, 124.89, 125.90, 128.45, 129.45, 129.63, 129.70, 134.32, 134.72, 135.10, 150.72, 150.82, 153.07, 160.37, 161.40, 162.81. HRMS (ESI): m/z 453.11683 (M+H+), 475.09903 (M+Na+), 491.07253 (M+K+).
3-Benzyl-4-methyl-2-oxo-2H-chromen-7-yl 4-methylbenzenesulfonate (76). Yield: 0.103 g, 49%, white solid, m.p.: 138–139 °C. 1H-NMR (CDCl3): δ 2.43 (s, 3H, CH3), 2.46 (s, 3H, CH3), 4.03 (s, 2H, CH2Ph), 6.82 (s, 1H, H-8), 7.08 (d, J = 8.8 Hz, 1H, H-6), 7.18–7.28 (m, 5H, CH2Ph), 7.33 (d, J = 8.0 Hz, 2H, PhSO3), 7.57 (d, J = 8.8 Hz, 1H, H-5), 7.72 (d, J = 8.0 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 15.54, 21.79, 33.02, 110.59, 118.80, 119.49, 125.57, 125.79, 126.50, 128.31, 128.48, 128.63, 130.07, 131.96, 138.48, 146.00, 146.65, 150.88, 152.55, 161.26. HRMS (ESI): m/z 421.11003 (M+H+), 443.09190 (M+Na+), 459.06602 (M+K+).
3-Benzyl-6-chloro-4-methyl-2-oxo-2H-chromen-7-yl 4-methylbenzenesulfonate (77). Yield: 0.136 g, 60%, white solid, m.p.: 173–174 °C. 1H-NMR (CDCl3): δ 2.41 (s, 3H, CH3), 2.48 (s, 3H, CH3), 4.04 (s, 2H, CH2Ph), 7.16 (s, 1H, H-8), 7.19–7.27 (m, 5H, CH2PhH), 7.36 (d, J = 8.0 Hz, 2H, ArH), 7.60 (s, 1H, H-5), 7.80 (d, J = 7.6 Hz, 2H, ArH). 13C-NMR (CDCl3): δ 15.54, 21.83, 33.11, 112.23, 120.28, 123.60, 126.10, 126.61, 126.68, 128.30, 128.63, 128.69, 130.10, 132.32, 138.17, 145.57, 146.26, 146.61, 150.81, 160.78. HRMS (ESI): m/z 455.07186 (M+H+).
3-Benzyl-4-ethyl-2-oxo-2H-chromen-7-yl 4-methylbenzenesulfonate (78). Yield: 0.169 g, 78%, white solid, m.p.: 66–68 °C. 1H-NMR (CDCl3): δ 1.13 (t, J = 7.6 Hz, 3H, CH2CH3), 2.46 (s, 3H, CH3), 2.85 (q, J = 7.6 Hz, 2H, CH2CH3), 4.01 (s, 2H, CH2Ph), 6.84 (d, J = 1.6 Hz, 1H, H-8), 7.07–7.10 (dd, J = 1.6, 8.8 Hz, 1H, H-6), 7.18–7.26 (m, 5H, CH2Ph), 7.34 (d, J = 8.0 Hz, 2H, PhSO3), 7.57 (d, J = 8.8 Hz, 1H, H-5), 7.74 (d, J = 8.0 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 18.14, 26.77, 27.26, 37.60, 115.83, 123.27, 123.83, 129.61, 130.74, 131.48, 133.22, 133.46, 133.60, 135.06, 137.05, 143.67, 150.98, 155.77, 157.18, 158.12, 166.55. HRMS (ESI): m/z 435.12593 (M+H+), 457.10761 (M+Na+), 473.08160 (M+K+).
3-Benzyl-2-oxo-4-propyl-2H-chromen-7-yl 4-methylbenzenesulfonate (79). Yield: 0.159 g, 80%, white solid, m.p.: 120 °C. 1H-NMR (CDCl3): δ 1.04 (t, J = 7.2 Hz, 3H, CH2CH3), 1.48 (q, J = 7.2 Hz, 3H, CH2CH3), 2.47 (s, 3H, CH3), 2.79 (q, J = 7.6 Hz, 2H, CH2CH2CH3), 4.02 (s, 2H, CH2Ph), 6.81 (s, 1H, H-8), 7.09 (d, J = 8.4 Hz, 1H, H-6), 7.19–7.25 (m, 5H, CH2Ph), 7.34 (d, J = 7.6 Hz, 2H, PhSO3), 7.55 (d, J = 8.8 Hz, 1H, H-5), 7.74 (d, J = 7.6 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 14.48, 21.80, 22.44, 31.15, 32.80, 110.80, 118.59, 118.84, 124.98, 125.83, 126.51, 128.21, 128.48, 128.61, 130.07, 132.04, 138.71, 145.99, 150.76, 150.86, 153.03, 161.54. HRMS (ESI): m/z 449.14190 (M+H+), 471.12381 (M+Na+), 487.09789 (M+K+).
4-Ethyl-3-(4-fluorobenzyl)-2-oxo-2H-chromen-7-yl 4-methylbenzenesulfonate (80). Yield: 0.132 g, 58%, white solid, m.p.: 96–97 °C. 1H-NMR (CDCl3): δ 1.15 (t, J = 7.2 Hz, 3H, CH2CH3), 2.47 (s, 2H, CH3), 2.85 (q, J = 7.2 Hz, 2H, CH2CH3), 3.97 (s, 2H, CH2Ph), 6.84 (s, 1H, H-8), 6.95 (t, J = 8.0 Hz, 2H, CH2Ph), 7.10 (d, J = 8.4 Hz, 1H, H-6), 7.21 (m, 2H, CH2Ph), 7.35 (t, J = 7.6 Hz, 2H, PhSO3), 7.59 (d, J = 8.8 Hz, 1H, H-5), 7.74 (d, J = 7.6 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 13.22, 21.80, 22.27, 31.86, 110.89, 115.29, 115.50, 118.17, 118.96, 124.45, 125.77, 128.47, 129.64, 129.72, 130.08, 132.04, 134.27, 134.30, 146.02, 150.85, 152.23, 153.12, 160.37, 161.50, 162.80. HRMS (ESI): m/z 453.11738 (M+H+).
3-(4-Fluorobenzyl)-4-methyl-2-oxo-2H-chromen-7-yl 4-methylbenzenesulfonate (81). Yield: 0.145 g, 66%, white solid, m.p.: 95–96 °C. 1H-NMR (CDCl3): δ 2.44 (s, 3H, CH3), 2.47(s, 3H, CH3), 3.99 (s, 2H, CH2Ph), 6.83 (s, 1H, H-8), 6.93–6.97 (m, J = 8.4 Hz, 2H, CH2Ph), 7.09 (d, J = 8.8 Hz, 1H, H-6), 7.20–7.22 (m, 2H, CH2Ph), 7.34 (d, J = 7.6 Hz, 2H, PhSO3), 7.58 (d, J = 8.4 Hz, 1H, H-5), 7.72 (d, J = 8.0 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 15.51, 21.79, 32.26, 110.64, 115.31, 115.52, 118.88, 119.38, 125.42, 125.83, 128.47, 129.72, 129.80, 130.07, 131.98, 134.08, 134.11, 146.02, 146.63, 150.95, 152.56, 160.36, 161.19, 162.79. HRMS (ESI): m/z 439.10127 (M+H+), 461.08292 (M+Na+), 477.05669 (M+K+).
3-(4-Fluorobenzyl)-2-oxo-4-propyl-2H-chromen-7-yl 4-methylbenzenesulfonate (82). Yield: 0.134 g, 58%, white solid, m.p.: 110–111 °C. 1H-NMR (CDCl3): δ 1.05 (t, J = 7.2 Hz, 3H, CH2CH3), 1.49–1.54 (m, J = 7.2 Hz, 2H, CH2CH2CH3), 2.47 (s, 3H, CH3), 2.79 (t, J = 7.6 Hz, 2H, CH2CH2CH3), 3.98 (s, 2H, CH2Ph), 6.83 (s, 1H, H-8), 6.93–6.97 (m, J = 6.8 Hz, 2H, CH2Ph), 7.10 (d, J = 8.8 Hz, 1H, H-6), 7.19–7.22 (m, 2H, CH2Ph), 7.35 (d, J = 7.2 Hz, 2H, PhSO3), 7.56 (d, J = 8.8 Hz, 1H, H-5), 7.74 (d, J = 6.8 Hz, 2H, PhSO3). 13C-NMR (CDCl3): δ 14.48, 21.80, 22.49, 31.14, 32.05, 110.84, 115.28, 115.50, 118.48, 118.90, 124.80, 125.87, 128.47, 129.62, 129.70, 130.07, 132.05, 134.31, 134.34, 146.01, 150.84, 150.89, 153.03, 160.37, 161.46, 162.80. HRMS (ESI): m/z 467.13229 (M+H+), 489.11399 (M+Na+), 505.08809 (M+K+).