Bolaamphiphiles Derived from Alkenyl L-Rhamnosides and Alkenyl D-Xylosides: Importance of the Hydrophilic Head
Abstract
:1. Introduction
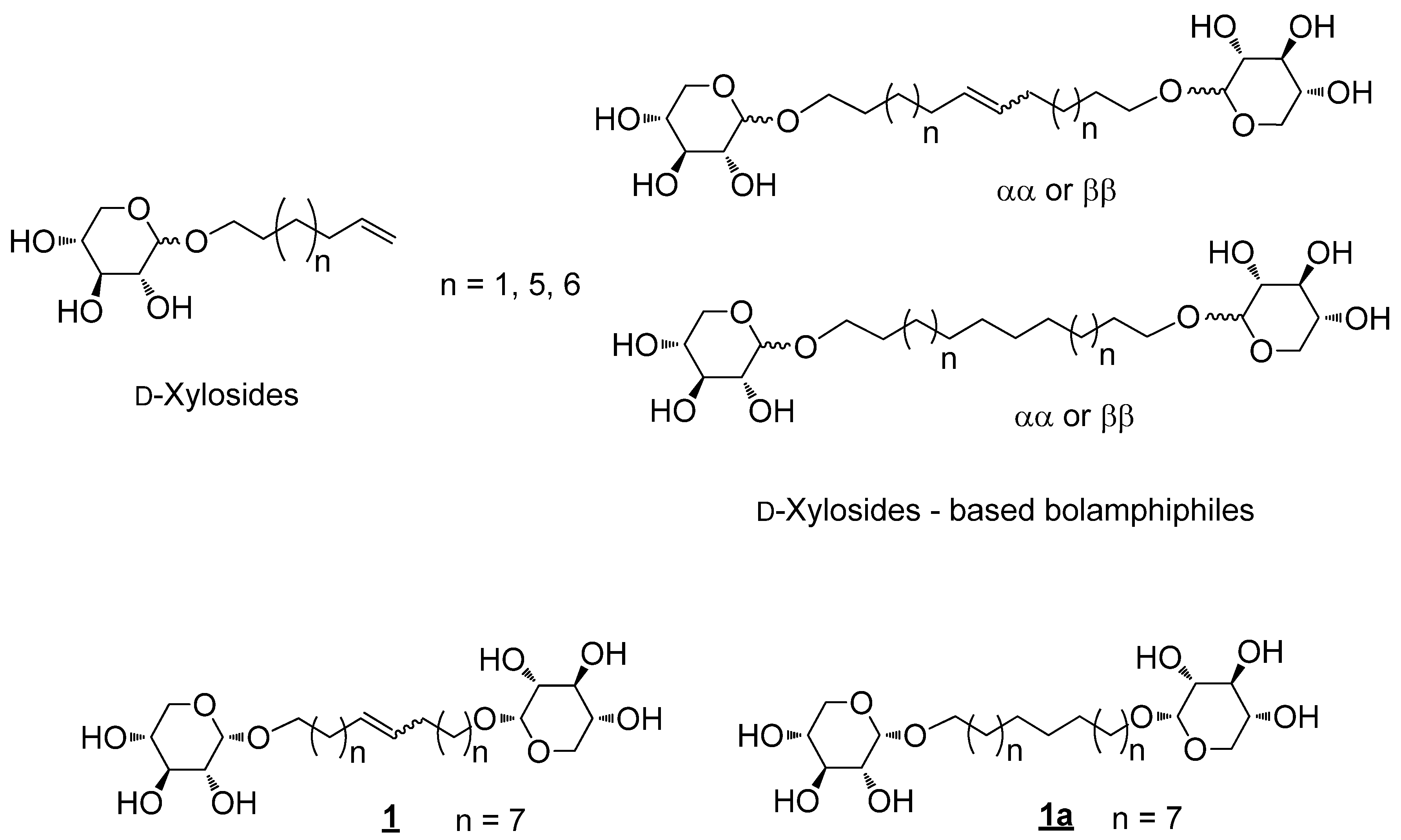
2. Results and Discussion
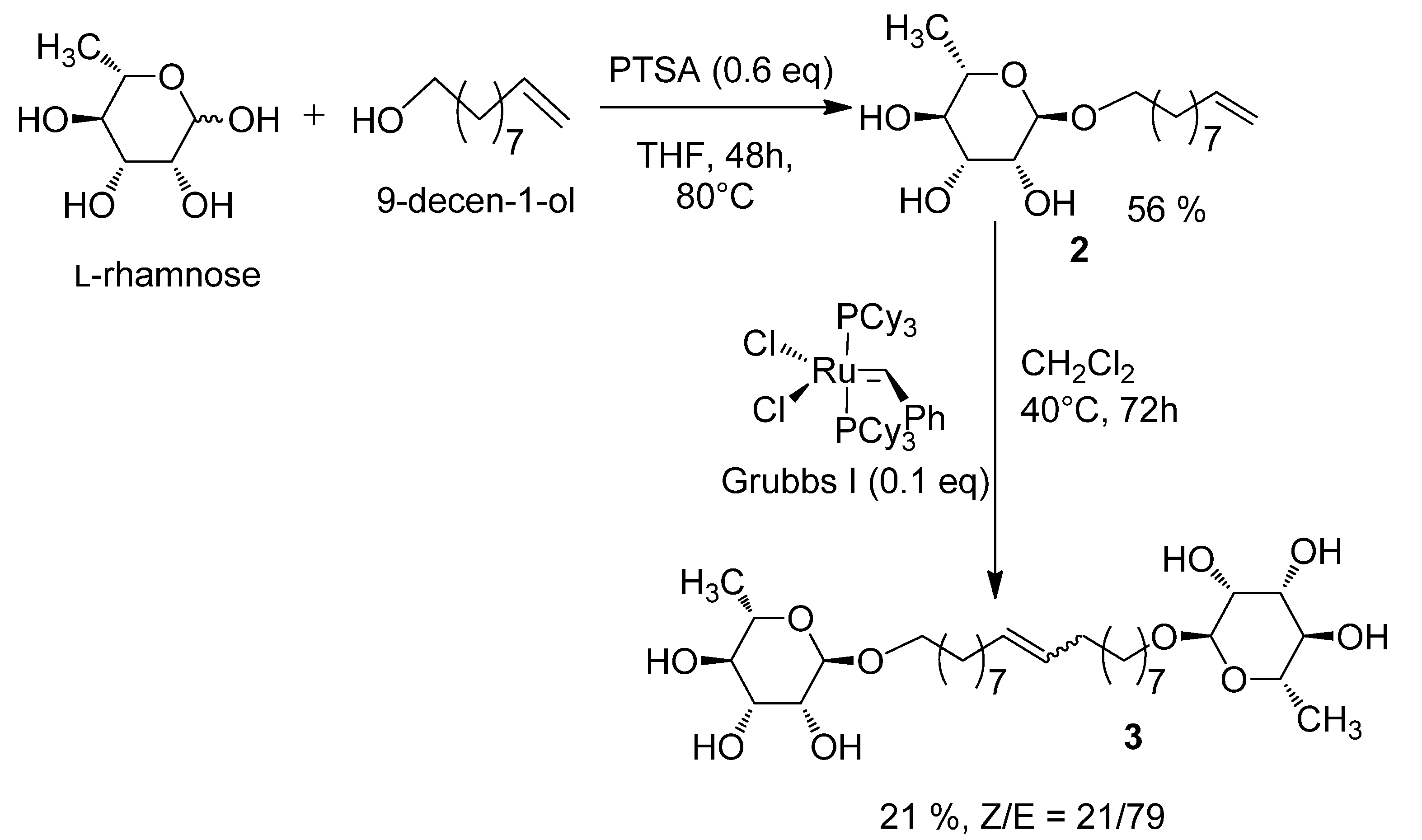
2.1. Synthesis of the 1', 18'-bis-octadec-9'-enyl-α-L-rhamnopyranoside (3)
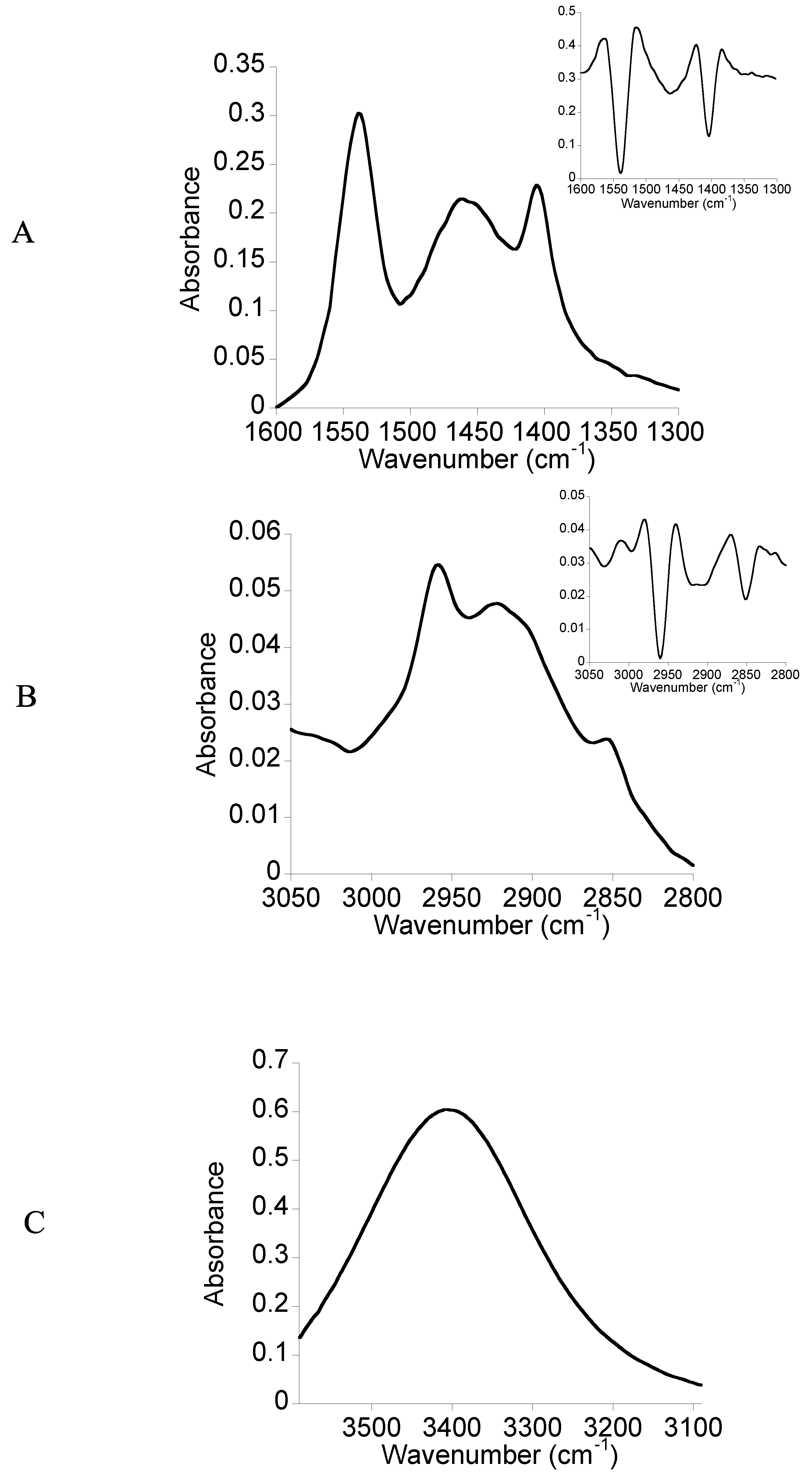
2.2. FTIR Spectroscopy
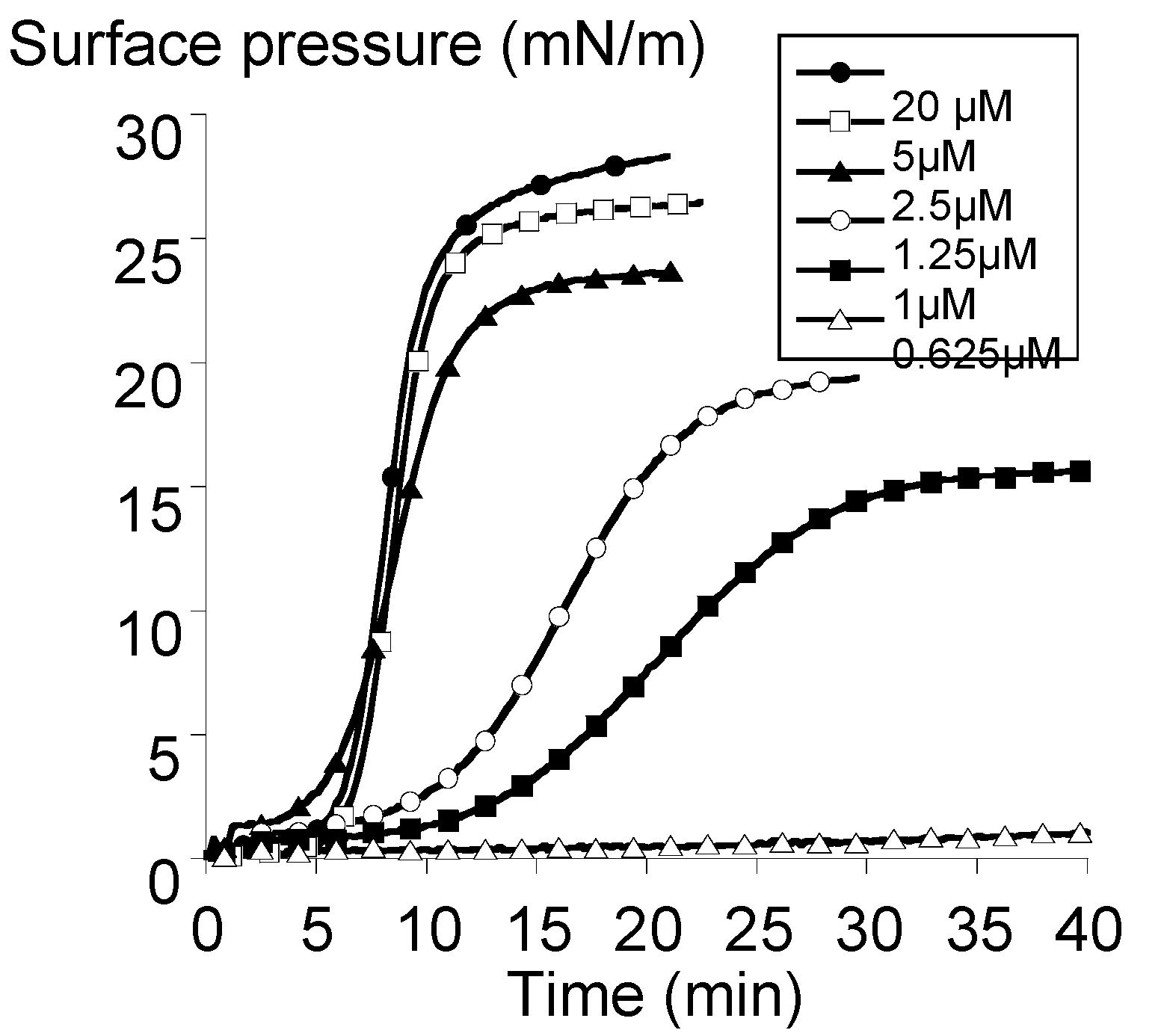
2.3. Surface Activity of the Rhamnoside-Based Bolaform (Bolaamphiphile 3)
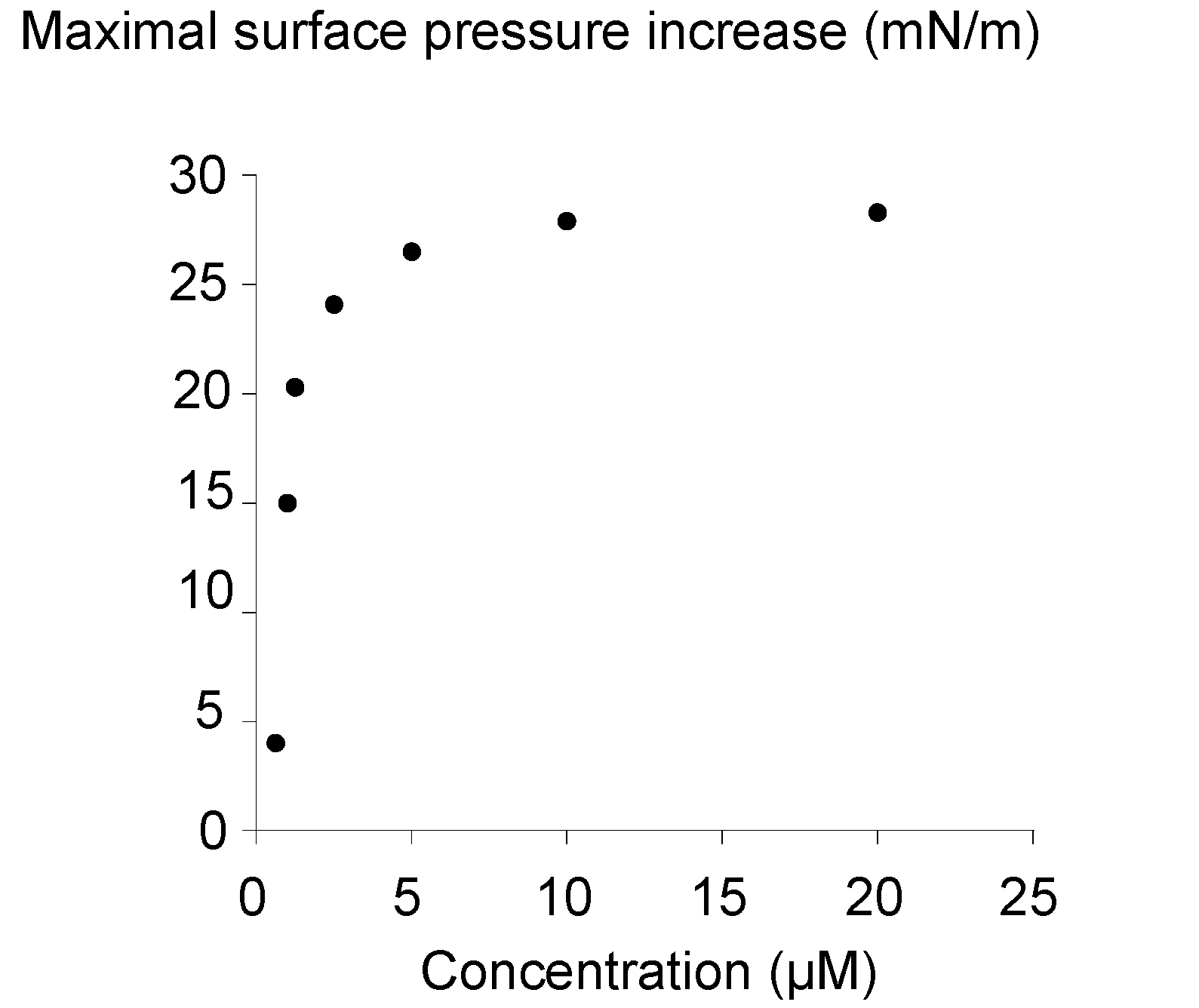
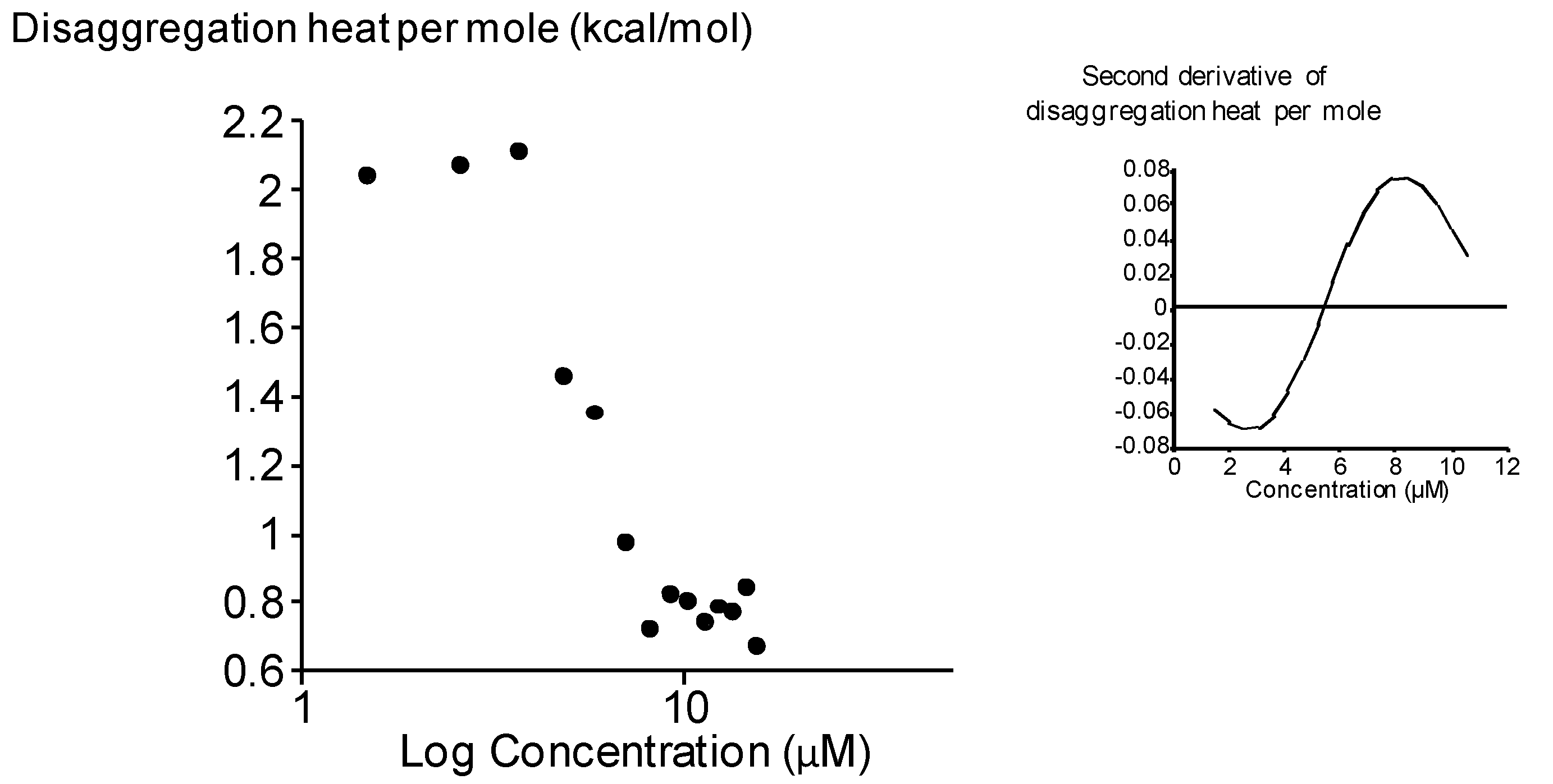
3. Experimental
3.1. General


3.2. Physico-Chemical Characterization
3.3. FTIR Spectroscopy
3.4. Adsorption Experiments at the Neat Air-Water Interface
3.5. Isothermal Titration Calorimetry (ITC) Experiments
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rojas, O.J.; Lucia, L.A.; Habibi, Y.; Stubenrauch, C. Biobased Surfactants and Detergents: Synthesis, Properties, and Applications; Hayes, D., Kitamoto, D., Solaiman, D., Ashby, R., Eds.; AOCS Press: Urbana, IL, USA, 2009. [Google Scholar]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Lang, S.; Wullbrandt, D. Rhamnose lipids–biosynthesis, microbial production and application potential. Appl. Microbiol. Biotechnol 1999, 51, 22–32. [Google Scholar]
- Soberón-Chávez, G.; Aguirre-Ramírez, M.; Sánchez, R. The Pseudomonas aeruginosa RhlA enzyme is involved in rhamnolipid and polyhydroxyalkanoate production. J. Ind. Microbiol. Biotechnol 2005, 32, 675–677. [Google Scholar]
- Nott, K.; Richard, G.; Laurent, P.; Jerome, C.; Blecker, C.; Wathelet, J.P.; Paquot, M.; Deleu, M. Enzymatic synthesis and surface properties of novel rhamnolipids. Process Biochem. 2013, 48, 133–143. [Google Scholar]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Ito, S.; Honda, H.; Tomita, F.; Suzuki, T. Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (mixture of C12, C13 and C14 fractions). J. Antibiot. 1971, 24, 855–859. [Google Scholar]
- Vatsa, P.; Sanchez, L.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010, 11, 5095–108. [Google Scholar]
- Varnier, A.-L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar]
- Deleu, M.; Damez, C.; Gatard, S.; Nott, K.; Paquot, M.; Bouquillon, S. Synthesis and physico-chemical characterization of bolaamphiphiles derived from alkenyl d-xylosides. New J. Chem. 2011, 35, 2258–2266. [Google Scholar]
- Deleu, M.; Gatard, S.; Payen, E.; Lins, L.; Nott, K.; Flore, C.; Thomas, R.; Paquot, M.; Bouquillon, S. d-xylose-based bolaamphiphiles: Synthesis and influence of the spacer nature on their interfacial and membrane properties. C.R. Chimie 2012, 15, 68–74. [Google Scholar]
- Damez, C.; Bouquillon, S.; Harakat, D.; Hénin, F.; Muzart, J.; Pezron, I.; Komunjer, L. Alkenyl and alkenoyl amphiphilic derivatives of d-xylose and their surfactant properties. Carbohydr. Res. 2007, 342, 154–162. [Google Scholar]
- Fischer, E. Über die Glucoside der Alkohole. Ber. Dtsch. Chem. Ges. 1893, 6, 2400–2412. [Google Scholar]
- Fischer, E.; Beensch, L. Ueber einige synthetische glucoside. Ber. Dtsch. Chem. Ges. 1894, 27, 2478–2486. [Google Scholar]
- Fischer, E. Ueber die verbindungen der zucker mit den alkoholen und ketonen. Ber. Dtsch. Chem. Ges. 1895, 28, 1145–1167. [Google Scholar]
- Jung, M.E.; Koch, P. An efficient synthesis of the protected carbohydrate moiety of Brasilicardin A. Org. Lett. 2011, 13, 3710–3713. [Google Scholar]
- Nasir, M.N.; Thawani, A.; Kouzayha, A.; Besson, F. Interactions of the natural antimicrobial mycosubtilin with phospholipid membrane models. Colloids Surf. B 2010, 78, 17–23. [Google Scholar]
- Volinsky, R.; Kolusheva, S.; Berman, A.; Jelinek, R. Microscopic visualization of alamethicin incorporation into model membrane monolayers. Langmuir 2004, 20, 11084–11091. [Google Scholar]
- Sun, Y.T.; Wang, S.X.; Sui, S.F. Penetration of human apolipoprotein H into air/water interface with and without phospholipid monolayers. Colloids Surf. A 2000, 175, 105–112. [Google Scholar]
- Pepper, K.G.; Bahrim, C.; Tadmor, R. Interfacial tension and spreading coefficient of thin films: Review and future directions. J. Adhes. Sci. Technol. 2011, 25, 1379–1391. [Google Scholar]
- Paddon-Jones, G.; Regismond, S.; Kwetkat, K.; Zana, R. Micellization of nonionic surfactant dimers and of the corresponding surfactant monomers in aqueous solution. J. Coll. Int. Sci. 2001, 243, 496–502. [Google Scholar]
- Nasir, M.N.; Besson, F. Conformational analyses of bacillomycin D, a natural antimicrobial lipopeptide, alone or in interaction with lipid monolayers at the air-water interface. J. Coll. Int. Sci. 2012, 387, 187–193. [Google Scholar]
- Sample Availability: Samples of the compound 1',18'-bis-octadec-9'-enyl-α-l-rhamnopyranoside are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gatard, S.; Nasir, M.N.; Deleu, M.; Klai, N.; Legrand, V.; Bouquillon, S. Bolaamphiphiles Derived from Alkenyl L-Rhamnosides and Alkenyl D-Xylosides: Importance of the Hydrophilic Head. Molecules 2013, 18, 6101-6112. https://doi.org/10.3390/molecules18056101
Gatard S, Nasir MN, Deleu M, Klai N, Legrand V, Bouquillon S. Bolaamphiphiles Derived from Alkenyl L-Rhamnosides and Alkenyl D-Xylosides: Importance of the Hydrophilic Head. Molecules. 2013; 18(5):6101-6112. https://doi.org/10.3390/molecules18056101
Chicago/Turabian StyleGatard, Sylvain, Mehmet Nail Nasir, Magali Deleu, Nadia Klai, Vincent Legrand, and Sandrine Bouquillon. 2013. "Bolaamphiphiles Derived from Alkenyl L-Rhamnosides and Alkenyl D-Xylosides: Importance of the Hydrophilic Head" Molecules 18, no. 5: 6101-6112. https://doi.org/10.3390/molecules18056101



