The Ameliorative Effect of Sophoricoside on Mast Cell-Mediated Allergic Inflammation in Vivo and in Vitro
Abstract
:1. Introduction
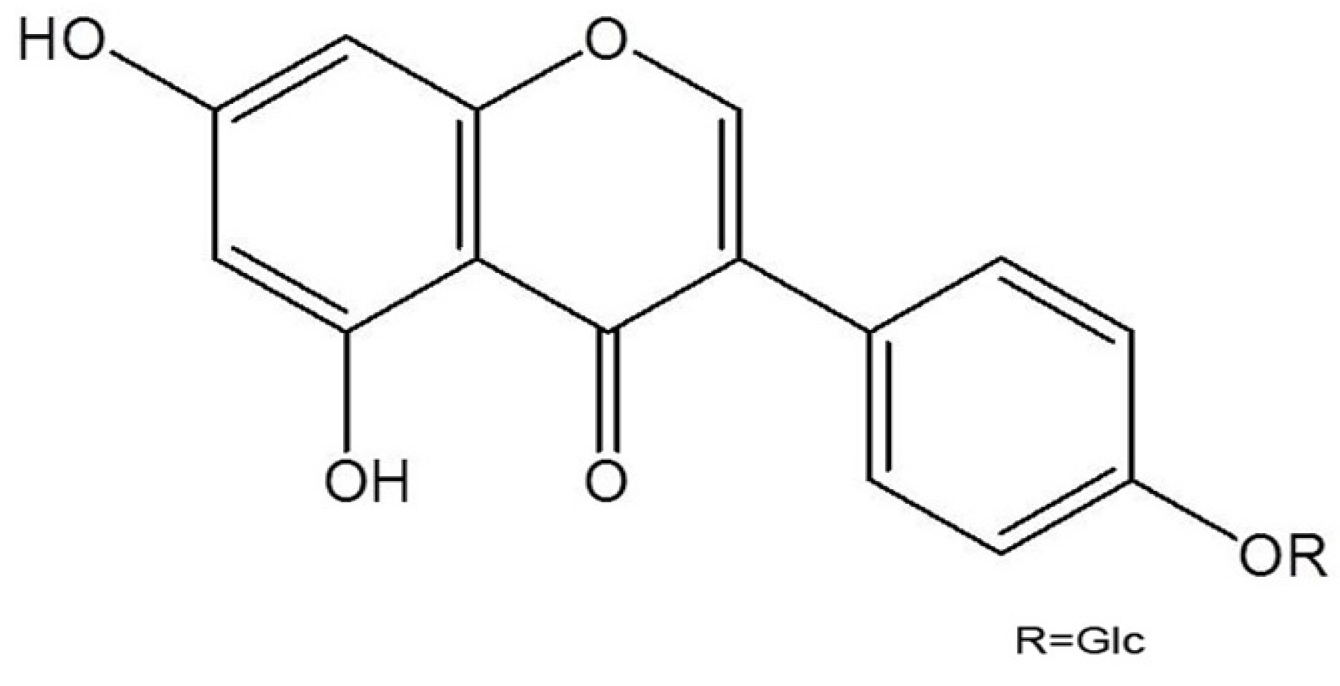
2. Results and Discussion
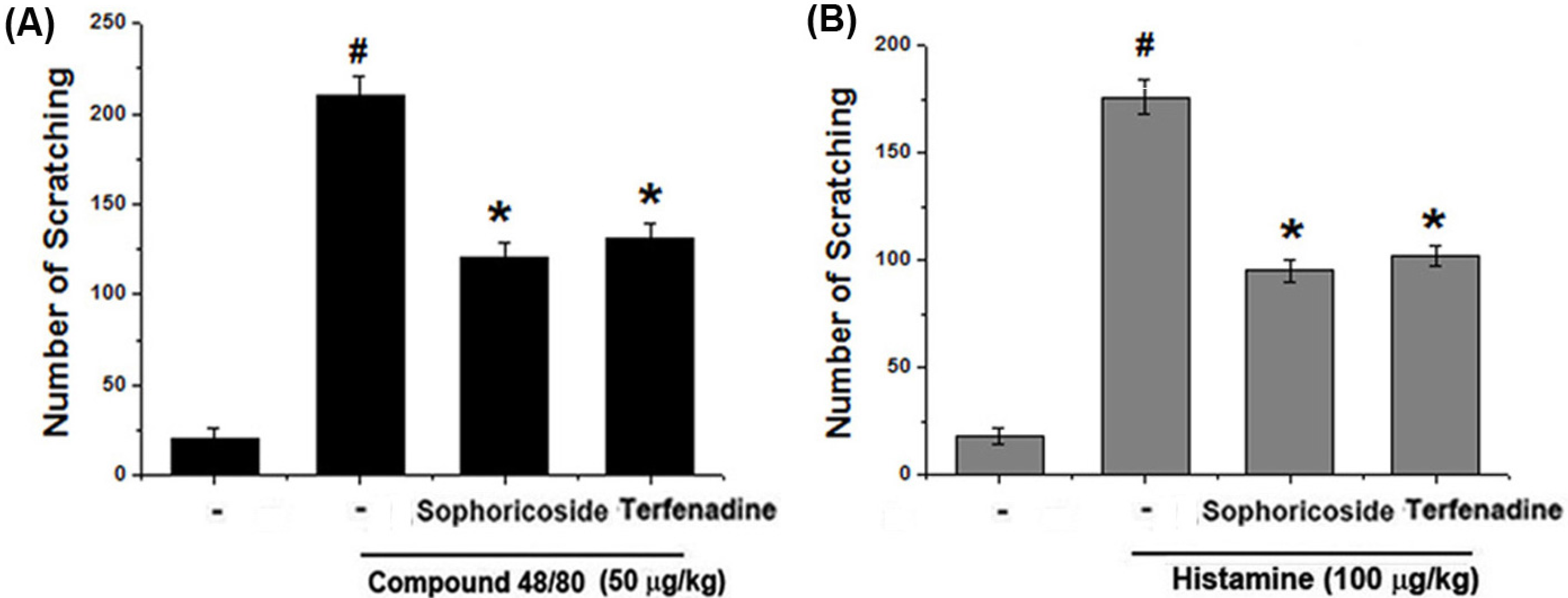
2.1. Effect of Sophoricoside on Scratching Behaviors in Mice
2.2. Effect of Sophoricoside on DNCB-Induced Atopic Dermatitis and IgE Levels in Serum

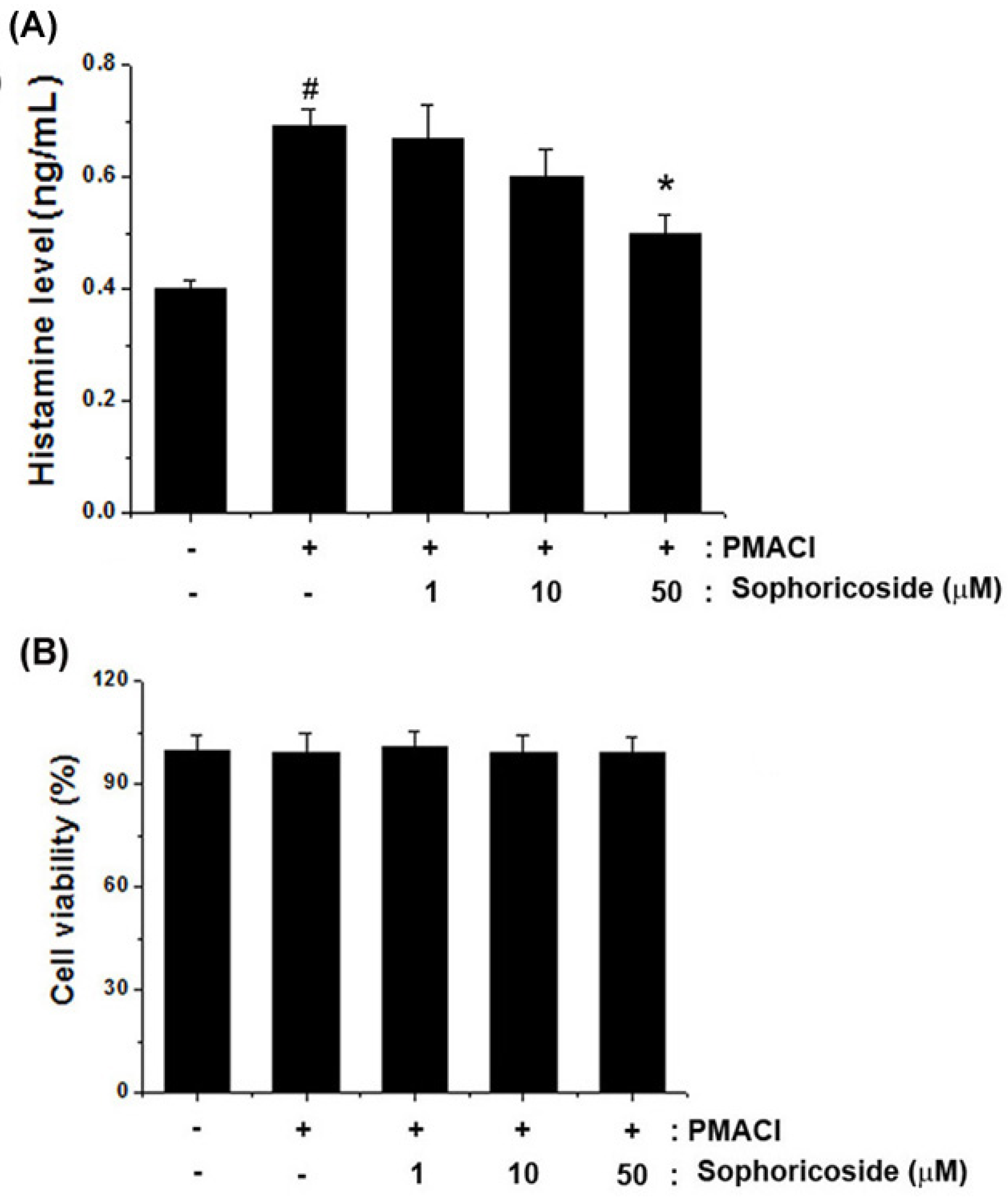
2.3. Effect of Sophoricoside on the Histamine Release in PMACI-Stimulated HMC-1 Cells
2.4. Effect of Sophoricoside on the Inflammatory Cytokines Production in PMACI-Stimulated HMC-1 Cells

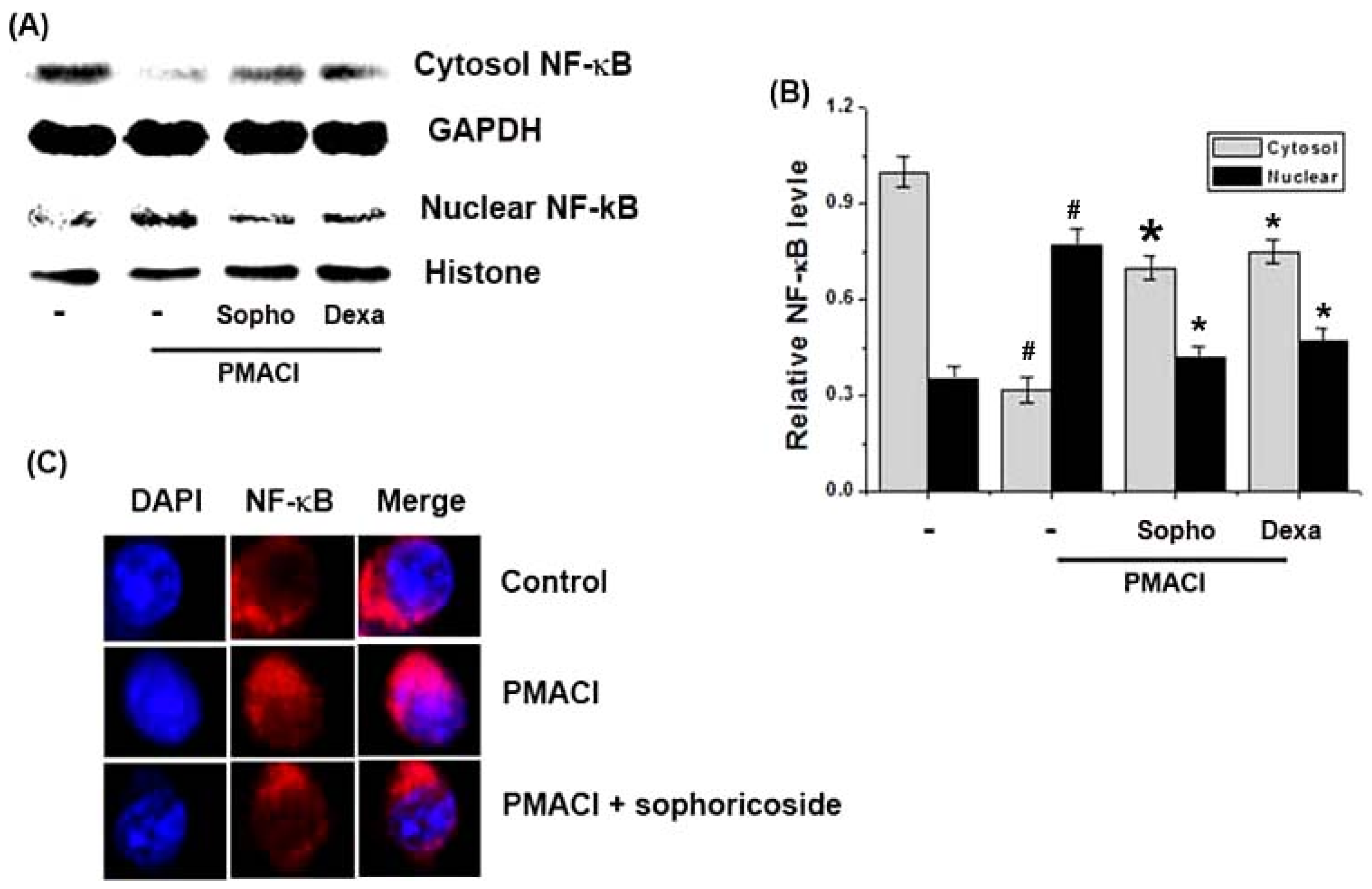
2.5. Effect of Sophoricoside on NF-κB Activation in the Nuclei of PMACI-Stimulated HMC-1 Cells
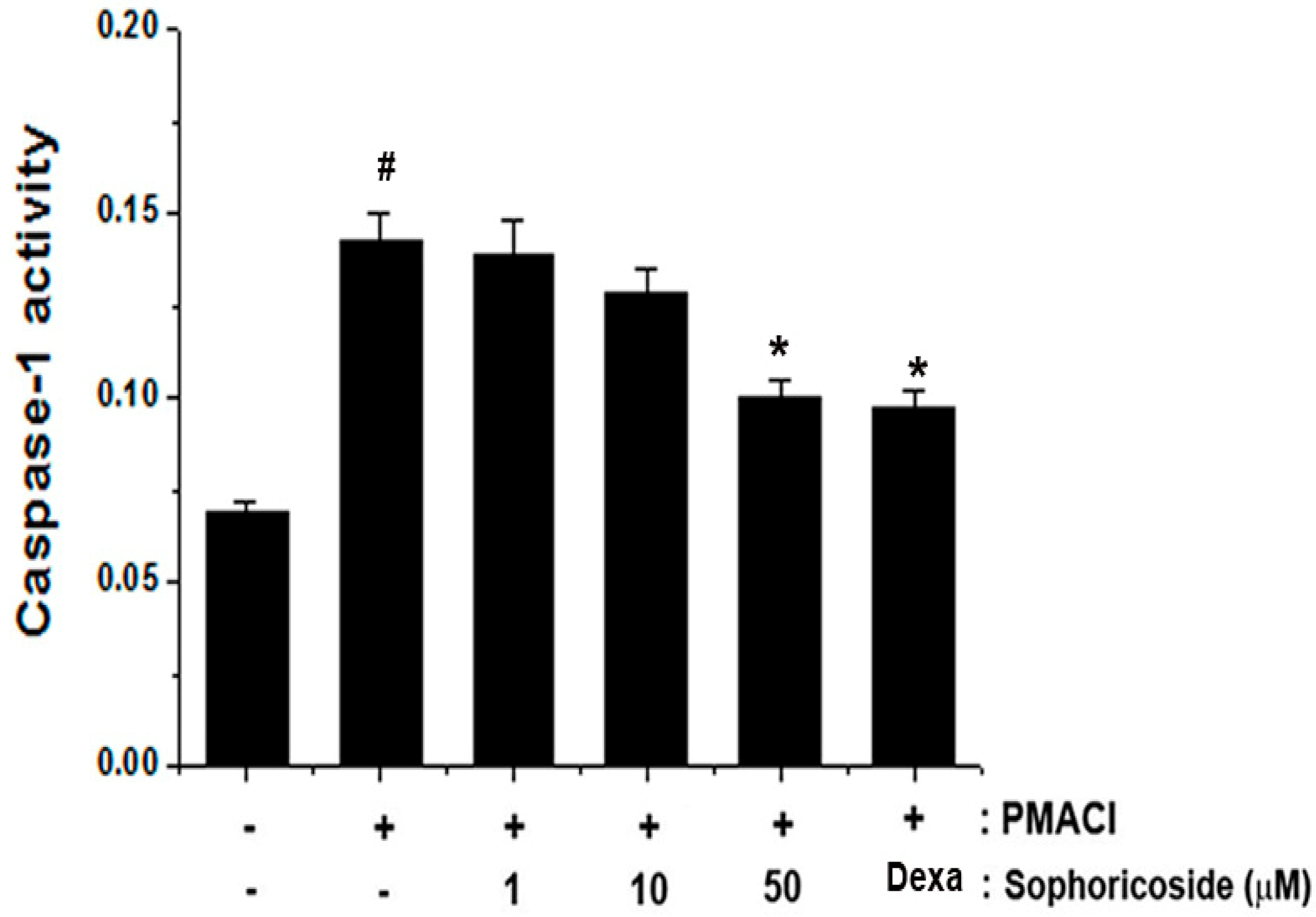
2.6. Effect of Sophoricoside on Caspase-1 Activation in PMACI-Stimulated HMC-1 Cells
2.7. Discussion

3. Experimental
3.1. Reagents
3.2. Isolation and Identification of Sophoricoside
3.3. Animals
3.4. Scratching Behavioral Experiment
3.5. DNCB-Induced Atopic Dermatitis
3.6. Cell Culture
3.7. MTT Assay
3.8. Histamine Assay
3.9. Assay of Cytokines
3.10. Preparation of Cytoplasmic and Nuclear Extract
3.11. Western Blot Analysis
3.12. NF-κB Immunofluorescence
3.13. Assay of Caspase-1 Activity
3.14. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Buske-Kirschbaum, A.; Geiben, A.; Hellhammer, D. Psychobiological aspects of atopic dermatitis: an overview. Psychother. Psychosom. 2001, 70, 6–16. [Google Scholar] [CrossRef]
- Gold, M.S.; Kemp, A.S. Atopic disease in childhood. Med. J. Aust. 2005, 182, 298–304. [Google Scholar]
- Trefzer, U.; Hofmann, M.; Sterry, W.; Asadullah, K. Cytokine and anti-cytokine therapy in dermatology. Expert Opin. Biol. Ther. 2003, 3, 733–743. [Google Scholar] [CrossRef]
- Tegeder, I.; Pfeilschifter, J.; Geisslinger, G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001, 15, 2057–2072. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Oldenburg, B.; Willemsen, E.C.; Spit, M.; Murzilli, S.; Salvatore, L.; Klomp, L.W.; Siersema, P.D.; van Erpecum, K.J.; van Mil, S.W. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim. Biophys. Acta 1812, 851–858. [Google Scholar]
- Mukaida, N. Interleukin-8: An expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 2000, 72, 391–398. [Google Scholar]
- Lee, S.H.; Stehlik, C.; Reed, J.C. Cop, a caspase recruitment domaincontaining protein and inhibitor of caspase-1 activation processing. J. Biol. Chem. 2001, 276, 34495–34500. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Figueroa, B.E.; Zhang, W.H.; Huo, C.; Guan, Y.; Zhang, Y.; Bruey, J.M.; Reed, J.C.; Friedlander, R.M. Dysregulation of receptor interacting protein-2 and caspase recruitment domain only protein mediates aberrant caspase-1 activation in huntington’s disease. J. Neurosci. 2005, 25, 11645–11654. [Google Scholar] [CrossRef]
- Faubel, S.; Lewis, E.C.; Reznikov, L.; Ljubanovic, D.; Hoke, T.S.; Somerset, H.; Oh, D.J.; Lu, L.; Klein, C.L.; Dinarello, C.A.; et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidne. J. Pharmacol. Exp. Ther. 2007, 322, 8–15. [Google Scholar] [CrossRef]
- Kim, B.H.; Chung, E.Y.; Min, B.K.; Lee, S.H.; Kim, M.K.; Min, K.R.; Kim, Y. Anti-inflammatory action of legume isoflavonoid sophoricoside through inhibition on cyclooxygenase-2 activity. Planta Med. 2003, 69, 474–476. [Google Scholar] [CrossRef]
- Fiedor, P.; Kozerski, L.; Dobrowolski, J.C.; Kawecki, R.; Biniecki, K.; Pachecka, J.; Rowiński, W.; Mazurek, A.P. Immunosuppressive effects of synthetic derivative of genistein on the survival of pancreatic islet allografts. Transplant. Proc. 1998, 30, 537–538. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; Ge, J.; Zhang, Y.; Wu, L.; Liu, J.; Zhang, X. Biotransformation of sophoricoside in Fructus sophorae by the fungus Schizophyllum commune. Bioresour. Technol. 2012, 111, 496–499. [Google Scholar] [CrossRef]
- Jung, SH.; Cho, S.H.; Dang, T.H.; Lee, J.H.; Ju, J.H.; Kim, M.K.; Lee, S.H.; Ryu, J.C.; Kim, Y. Structuralrequirement of isoflavonones for the inhibitoryactivity of interleukin-5. Eur. J. Med. Chem. 2003, 38, 537–545. [Google Scholar]
- Du, N.; Xu, Y.; Chen, W.Z.; Zhang, F.H. Effect of Sophoricoside on histomorphology of bone in ovariectomized rats. Zhong Xi Yi Jie He Xue Bao 2003, 1, 44–46. [Google Scholar] [CrossRef]
- Humke, E.W.; Shriver, S.K.; Starovasnik, M.A.; Fairbrother, W.J.; Dixit, V.M. ICEBERG: A novel inhibitor of interleukin-1β generation. Cell 2000, 103, 99–111. [Google Scholar]
- Leung, D.Y.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- Shiohara, T.; Hayakawa, J.; Mizukawa, Y. Animal models for atopic dermatitis: Are they relevant to human disease? J. Dermatol. Sci. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Allam, J.P.; Novak, N. The pathophysiology of atopic eczema. Clin. Exp. Dermatol. 2006, 31, 89–93. [Google Scholar]
- Brenninkmeijer, E.E.; Spuls, P.I.; Legierse, C.M.; Lindeboom, R.; Smitt, J.H.; Bos, J.D. Clinical differences between atopic and atopiform dermatitis. J. Am. Acad. Dermatol. 2008, 58, 407–414. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, H.S.; Kim, Y.J.; Kim, J.S.; Park, Y.S.; Kang, J.S.; Yuk, D.Y.; Hong, J.T.; Kim, Y.; Han, S.B. Sophoricoside isolated from Sophora japonica ameliorates contact dermatitis by inhibiting NF-κB signaling in B cells. Int. Immunopharmacol. 2013, 15, 467–473. [Google Scholar]
- Minami, K.; Kamei, C. A chronic model for evaluating the itching associated with allergic conjunctivitis in rats. Int. Immunopharmacol. 2004, 4, 101–108. [Google Scholar] [CrossRef]
- Harvima, I.T.; Nilsson, G. Mast cells as regulators of skin inflammation and immunity. Acta Derm. Venereol. 2011, 91, 644–650. [Google Scholar]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Bunikowski, R.; Gerhold, K.; Bräutigam, M.; Hamelmann, E.; Renz, H.; Wahn, U. Effect of low-dose cyclosporin a microemulsion on disease severity, interleukin-6, interleukin-8 and tumor necrosis factor alpha production in severe pediatric atopic dermatiti. Int. Arch. Allergy Immunol. 2001, 125, 344–348. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Garbati, M.R. Inhibition of NF-κB signaling as a strategy in disease therapy. Curr. Top. Microbiol. Immunol. 2011, 349, 245–263. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kalai, M.; Saelens, X.; Declercq, W.; Vandenabeele, P. Caspase-1 activates nuclear factor of the kappaenhancer in B cells independently of its enzymatic activity. J. Biol. Chem. 2004, 279, 24785–24793. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Umakoshi, K.; Nojiri, N.; Kamei, C. Effect of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur. J. Pharmacol. 1998, 351, 1–5. [Google Scholar] [CrossRef]
- Gao, X.K.; Fuseda, K.; Shibata, T.; Tanaka, H.; Inagaki, N.; Nagai, H. Kampo medicines for mite antigen-induced allergic dermatitis in NC/Nga mice. Evid. Based. Complement Alterna. Med. 2005, 2, 191–199. [Google Scholar] [CrossRef]
- Schoonbroodt, S.; Legrand-Poels, S.; Best-Belpomme, M.; Piette, J. Activation of the NF-kappaB transcription factor in a T-lymphocytic cell line by hypochlorous acid. Biochem. J. 1997, 321, 777–785. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, S.-J.; Lee, G.-Y.; Jung, J.-W.; Oh, S.-R.; Ahn, E.-M.; Kim, S.-H.; Hong, S.-H.; Um, J.-Y. The Ameliorative Effect of Sophoricoside on Mast Cell-Mediated Allergic Inflammation in Vivo and in Vitro. Molecules 2013, 18, 6113-6127. https://doi.org/10.3390/molecules18056113
Kim S-J, Lee G-Y, Jung J-W, Oh S-R, Ahn E-M, Kim S-H, Hong S-H, Um J-Y. The Ameliorative Effect of Sophoricoside on Mast Cell-Mediated Allergic Inflammation in Vivo and in Vitro. Molecules. 2013; 18(5):6113-6127. https://doi.org/10.3390/molecules18056113
Chicago/Turabian StyleKim, Su-Jin, Gil-Yong Lee, Ji-Wook Jung, Sa-Rang Oh, Eun-Mi Ahn, Sung-Hoon Kim, Seung-Heon Hong, and Jae-Young Um. 2013. "The Ameliorative Effect of Sophoricoside on Mast Cell-Mediated Allergic Inflammation in Vivo and in Vitro" Molecules 18, no. 5: 6113-6127. https://doi.org/10.3390/molecules18056113




