Phenolic Glucosides from Dendrobium aurantiacum var. denneanum and Their Bioactivities
Abstract
:1. Introduction
2. Results and Discussion
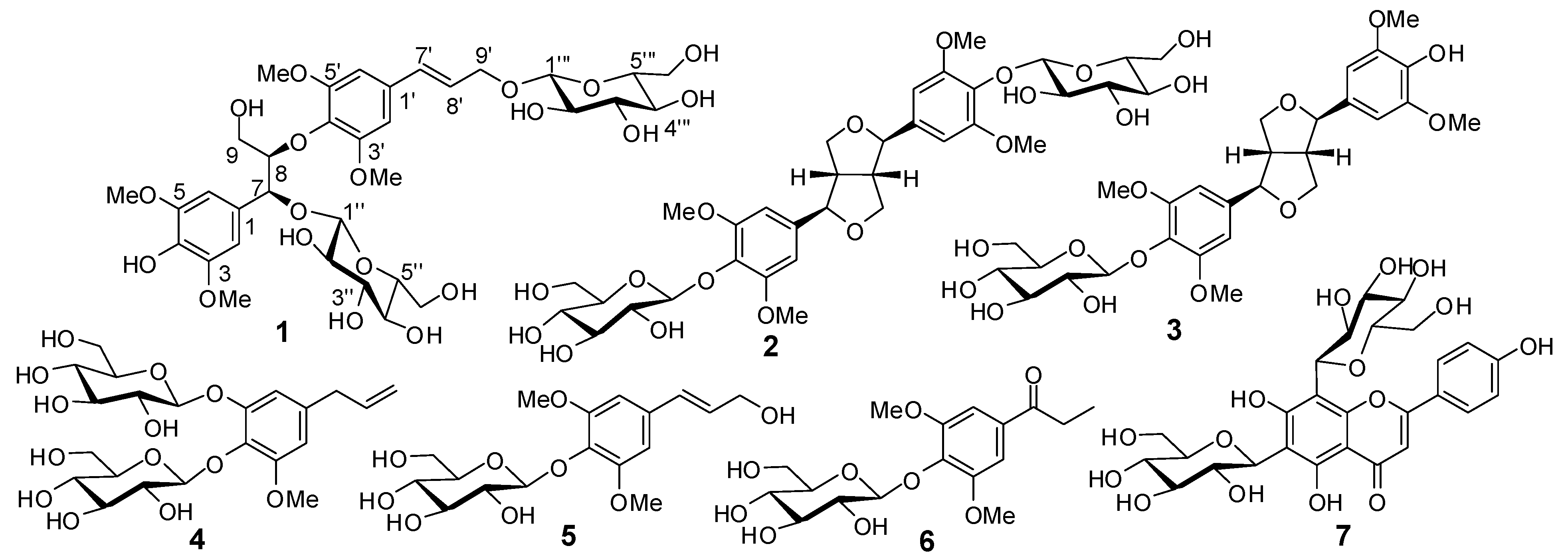
| No. | δH | δC | No. | δH | δC |
|---|---|---|---|---|---|
| 1 | – | 129.8 | 1'' | 4.34 d (7.5) | 103.1 |
| 2 | 6.72 s | 106.1 | 2'' | 3.18 m | 75.2 |
| 3 | – | 148.2 | 3'' | 3.06 m | 77.8 |
| 4 | – | 135.6 | 4'' | 3.03 m | 71.1 |
| 5 | – | 148.2 | 5'' | 3.02 m | 78.3 |
| 6 | 6.72 s | 106.1 | 6''a | 3.66 (overlapped) | 62.1 |
| 7 | 5.11 d (3.5) | 79.5 | 6''b | 3.41 (overlapped) | – |
| 8 | 4.27 m | 85.0 | 1''' | 4.22 d (8.0) | 103.2 |
| 9a | 3.59 m | 61.2 | 2''' | 3.00 m | 74.5 |
| 9b | 3.17 m | – | 3''' | 3.13 m | 77.4 |
| 1' | – | 133.1 | 4''' | 3.12 m | 70.9 |
| 2' | 6.77 s | 104.8 | 5''' | 3.23 m | 77.9 |
| 3' | – | 153.7 | 6'''a | 3.62 (overlapped) | 61.9 |
| 4' | – | 136.7 | 6'''b | 3.40 (overlapped) | |
| 5' | – | 153.7 | 3/5-OMe | 3.73 s | 57.0 |
| 6' | 6.77 s | 104.8 | 3'/5'-OMe | 3.78 s | 57.0 |
| 7' | 6.59 d (16.0) | 132.2 | |||
| 8' | 6.35 dt (16.0, 6.0) | 126.8 | |||
| 9'a | 4.43 dd (13.0, 6.0) | 69.6 | |||
| 9'b | 4.20 dd (13.0, 6.0) | – |

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
 = −12.2 (c = 0.20, MeOH); IR (KBr) νmax: 3374, 2920, 1588, 1502, 1462, 1420, 1331, 1226, 1124, 1071, 1023, 835, 706, 615 cm−1; UV (MeOH) λmax (log ε): 204 (4.35), 226 (4.03, sh), 271 (3.67) nm; CD (MeOH): 222 (Δε +0.16), 235 (Δε −0.18), 251 (Δε +0.03), 266 (Δε −0.12), 290 (Δε +0.23) nm; ESI-MS m/z783 [M+Na]+; HRESI-MS: m/z 783.2681 [M+Na]+ (calcd for C34H48O19Na, 783.2687); 1H- and 13C-NMR data see Table 1.
= −12.2 (c = 0.20, MeOH); IR (KBr) νmax: 3374, 2920, 1588, 1502, 1462, 1420, 1331, 1226, 1124, 1071, 1023, 835, 706, 615 cm−1; UV (MeOH) λmax (log ε): 204 (4.35), 226 (4.03, sh), 271 (3.67) nm; CD (MeOH): 222 (Δε +0.16), 235 (Δε −0.18), 251 (Δε +0.03), 266 (Δε −0.12), 290 (Δε +0.23) nm; ESI-MS m/z783 [M+Na]+; HRESI-MS: m/z 783.2681 [M+Na]+ (calcd for C34H48O19Na, 783.2687); 1H- and 13C-NMR data see Table 1.3.4. Enzymatic Hydrolysis of 1
 = +13.2 (c = 0.02, MeOH); CD (MeOH) 221 (Δε +1.25), 239 (Δε −0.22), 270 (Δε +0.43) nm; 1H-NMR (CD3OD, 500 MHz) δ: 6.76 (2H, s, H-2', 6'), 6.68 (2H, s, H-2, 6), 6.55 (1H, d, J = 16.0 Hz, H-7'), 6.38 (1H, dt, J = 16.0, 6.0 Hz, H-8'), 4.99 (1H, d, J = 3.5 Hz, H-7), 4.27 (2H, d, J = 6.0 Hz, H2-9'), 4.19 (1H, m, H-8), 3.88 and 3.83 (each 6H, s, OMe-3, 5, 3', 5'), 3.87 (1H, m, H-9a), 3.48 (1H, m, H-9b); ESI-MS m/z: 459 [M+Na]+; HR-ESI-MS m/z: 459.1624 [M+Na]+ (calcd for C22H28O9Na, 459.1631).
= +13.2 (c = 0.02, MeOH); CD (MeOH) 221 (Δε +1.25), 239 (Δε −0.22), 270 (Δε +0.43) nm; 1H-NMR (CD3OD, 500 MHz) δ: 6.76 (2H, s, H-2', 6'), 6.68 (2H, s, H-2, 6), 6.55 (1H, d, J = 16.0 Hz, H-7'), 6.38 (1H, dt, J = 16.0, 6.0 Hz, H-8'), 4.99 (1H, d, J = 3.5 Hz, H-7), 4.27 (2H, d, J = 6.0 Hz, H2-9'), 4.19 (1H, m, H-8), 3.88 and 3.83 (each 6H, s, OMe-3, 5, 3', 5'), 3.87 (1H, m, H-9a), 3.48 (1H, m, H-9b); ESI-MS m/z: 459 [M+Na]+; HR-ESI-MS m/z: 459.1624 [M+Na]+ (calcd for C22H28O9Na, 459.1631).3.5. Cell Culture and Assessment of Cytotoxic Activity against Human Tumor Cells
3.6. Cell Culture and Assessment of Neuroprotective Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Commission of Chinese Pharmacopoeia, Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 85–87.
- Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Publishing House: Shanghai, China, 1995; pp. 586–590.
- Zheng, W.P.; Tang, Y.P.; Lou, F.C.; Zhi, F. Studies on the constituents of Dendrobium chryseum Rolfe. J. China Pharm. Univ. 2000, 31, 5–7. [Google Scholar]
- Yang, L.; Wang, Z.T.; Xu, L.S. Phenols and a triterpene from Dendrobium aurantiacum var. denneanum (Orchidaceae). Biochem. Syst. Ecol. 2006, 34, 658–660. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.H.; Zhang, Y.; Chen, Y.G. Chemical constituents of Dendrobium aurantiacum var. denneanum. Chem. Nat. Compd. 2009, 45, 525–527. [Google Scholar] [CrossRef]
- Yang, L.; Han, H.F.; Nakamura, N.; Hattori, M.; Wang, Z.T.; Xu, L.S. Bio-guided isolation of antioxidants from the stems of Dendrobium aurantiacum var. denneanum. Phytother. Res. 2007, 21, 696–698. [Google Scholar] [CrossRef]
- Moss, G.P. Nomenclature of lignans and neolignans (IUPAC recommendations 2000). Pure Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef]
- El Gamal, A.A.; Takeya, K.; Itokawa, H.; Halim, A.F.; Amer, M.M.; Saad, H.E.A. Lignan bis-glucosides from Galium sinaicum. Phytochemistry 1997, 45, 597–600. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yu, D.Q. Lignan and acetylenic glycosides from Aster auriculatus. Phytochemistry 1998, 48, 711–717. [Google Scholar] [CrossRef]
- Kuang, H.X.; Shao, C.J.; Kasai, R.; Ohtani, K.; Tian, Z.K.; Xu, J.D.; Tanaka, O. Phenolic glycosides from roots of Adenophora tetraphylla collected in Heilongjiang, China. Chem. Pharm. Bull. 1991, 39, 2440–2442. [Google Scholar] [CrossRef]
- Sugiyama, M.; Nagayama, E.; Kikuchi, M. Lignan and phenylpropanoid glycosides from Osmanthus aslaticus. Phytochemistry 1993, 33, 1215–1219. [Google Scholar] [CrossRef]
- Takara, K.; Matsui, D.; Wada, K.; Ichiba, T.; Chinen, I.; Nakasone, Y. New phenolic compounds from Kokuto, non-centrifuged cane sugar. Biosci. Biotechnol. Biochem. 2003, 67, 376–379. [Google Scholar] [CrossRef]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. A. Study of flavonoids of sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food. Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Wu, X. New lignan glucosides with tyrosinase inhibitory activities from exocarp of Castanea henryi. Carbohydr. Res. 2012, 355, 45–49. [Google Scholar] [CrossRef]
- Lourith, N.; Katayama, T.; Suzuki, T. Stereochemistry and biosynthesis of 8-O-4' neolignans in Eucommia ulmoides: diastereoselective formation of guaiacylglycerol-8-O-4'-(sinapyl alcohol) ether. J. Wood Sci. 2009, 51, 370–378. [Google Scholar] [CrossRef]
- Hudson, C.S.; Dale, J.K. Studies on the forms of D-glucose and their mutarotation. J. Am. Chem. Soc. 1917, 39, 320–328. [Google Scholar] [CrossRef]
- Zi, J.C.; Li, S.; Liu, M.T.; Gan, M.L.; Lin, S.; Song, W.X.; Zhang, Y.L.; Fan, X.N.; Yang, Y.C.; Zhang, J.J.; Shi, J.G.; Di, D.L. Glycosidic constituents of the tubers of Gymnadenia conopsea. J. Nat. Prod. 2008, 71, 799–805. [Google Scholar] [CrossRef]
- Gan, M.L.; Zhang, Y.L.; Lin, S.; Liu, M.T.; Song, W.X.; Zi, J.C.; Yang, Y.C.; Fan, X.N.; Shi, J.G.; Hu, J.F.; Sun, J.D.; Chen, N.H. Glycosides from the root of Iodes cirrhosa. J. Nat. Prod. 2008, 71, 647–654. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, H.K.; Choi, S.U.; Moon, E.; Kim, S.Y.; Lee, K.R. Bioactive lignans from the rhizomes of Acorus gramineus. J. Nat. Prod. 2011, 74, 2187–2192. [Google Scholar] [CrossRef]
- Fang, J.M.; Lee, C.K.; Cheng, Y.S. Lignans from leaves of Juniperus chinensis. Phytochemistry 1992, 31, 3659–3661. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef]
- Ye, Q.H.; Zhao, W.M.; Qin, G.W. Lignans from Dendrobium chrysanthum. J. Asian Nat. Prod. Res. 2004, 6, 39–43. [Google Scholar] [CrossRef]
- Ito, M.; Matsuzaki, K.; Wang, J.; Daikonya, A.; Wang, N.L.; Yao, X.S.; Kitanaka, S. New phenanthrenes and stilbenes from Dendrobium loddigesii. Chem. Pharm. Bull. 2010, 58, 628–633. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.K.; Wang, N.L.; Kurihara, H.; Yao, X.S. Antioxidant phenanthrenes and lignans from Dendrobium nobile. J. Chin. Pharm. Sci. 2008, 17, 314–318. [Google Scholar]
- Zhao, W.M.; Ye, Q.H.; Dai, J.Q.; Martin, M.T.; Zhu, J.P. allo-Aromadendrane- and picrotoxane-type sesquiterpenes from Dendrobium moniliforme. Planta Med. 2003, 69, 1136–1140. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.F.; Wang, Z.T.; Xu, L.S. Studies on chemical constituents of Dendrobium trigonopus Rchb. f. Chin. J. Nat. Med. 2005, 3, 28–30. [Google Scholar]
- Shao, L.; Huang, W.H.; Zhang, C.F.; Wang, L.; Zhang, M.; Wang, Z.T. Study on chemical constituents from stem of Dendrobium aphyllum. Zhongguo Zhongyao Zazhi 2008, 33, 1693–1695. [Google Scholar]
- Zhang, X.; Gao, H.; Wang, N.L.; Yao, X.S. Phenolic components from Dendrobium nobile. Zhongcaoyao 2006, 37, 652–655. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiong, L.; Cao, Z.-X.; Peng, C.; Li, X.-H.; Xie, X.-F.; Zhang, T.-M.; Zhou, Q.-M.; Yang, L.; Guo, L. Phenolic Glucosides from Dendrobium aurantiacum var. denneanum and Their Bioactivities. Molecules 2013, 18, 6153-6160. https://doi.org/10.3390/molecules18066153
Xiong L, Cao Z-X, Peng C, Li X-H, Xie X-F, Zhang T-M, Zhou Q-M, Yang L, Guo L. Phenolic Glucosides from Dendrobium aurantiacum var. denneanum and Their Bioactivities. Molecules. 2013; 18(6):6153-6160. https://doi.org/10.3390/molecules18066153
Chicago/Turabian StyleXiong, Liang, Zhi-Xing Cao, Cheng Peng, Xiao-Hong Li, Xiao-Fang Xie, Ting-Mo Zhang, Qin-Mei Zhou, Lian Yang, and Li Guo. 2013. "Phenolic Glucosides from Dendrobium aurantiacum var. denneanum and Their Bioactivities" Molecules 18, no. 6: 6153-6160. https://doi.org/10.3390/molecules18066153




