Design, Synthesis and Evaluation of Dual-Modality Glyco-Nanoparticles for Tumor Imaging
Abstract
:1. Introduction
2. Results and Discussion
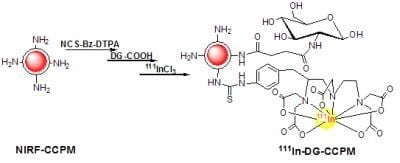
2.1. Synthesis and Characterization of CCPM Nanoparticles

| Specification | NIRF-CCPM | DTPA-NIRF-CCPM | DG-CCPM |
|---|---|---|---|
| Particles (nm) a | 24 ± 8.9 | 24 ± 8.9 | 24 ± 8.9 |
| Zeta potential (mV) b | +1.2 | −5.83 ± 2.40 | −5.83 ± 1.08 |
| Dyes/Particle c | 21 | 21 | 21 |
| DTPA/Particle d | 0 | 19 | 19 |
| Amines/Particle e | 180 | 160 | 145 |
| Concentration f | 1.54 × 1015 | 7 × 1014 | 3.4 × 1014 |
| DG/Particle g | N/A | N/A | 15 |
2.2. Radiolabeling of the Nanoparticles

2.3. Microscopic Imaging of DTPA-NIRF-CCPM-DG
2.4. Biodistribution in Tumor Bearing mice



2.5. In Vivo Gamma Imaging

2.6. In vivo NIRF Imaging

3. Experimental
3.1. General
3.2. Synthesis and Characterization of DG-CCPM
3.2.1. Synthesis of d-Glucosamine-NH-SA
3.2.2. Conjugation of DG-COOH to CCPM

3.2.3. Standard Curves of DTPA-NIRF-CCPM
3.3. Radiolabeling

3.4. Fluorescence Microscopy Study
3.5. Biodistribution Studies
3.6. In Vivo Gamma Imaging
3.7. In Vivo NIRF Imaging
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ntziachristos, V.; Bremer, C.; Weissleder, R. Fluorescence imaging withnear-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003, 13, 195–208. [Google Scholar]
- Gurfinkel, M.; Ke, S.; Wen, X.; Li, C.; Sevick-Muraca, E.M. Near-infrared fluorescence optical imaging and tomography. Dis. Markers 2003, 19, 107–121. [Google Scholar]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumor itropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Torchilin, V.P. Targeted pharmaceutical nano carriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar]
- Moore, A.; Medarova, Z.; Potthast, A.; Dai, G. In Vivo targeting of underglycosylatedmuc-1 tumor antigen using a multimodal imaging probe. Cancer Res. 2004, 64, 1821–1827. [Google Scholar] [CrossRef]
- Lisy, M.R.; Hartung, A.; Lang, C.; Schuler, D.; Richter, W.; Reichenbach, J.R.; Kaiser, W.A.; Hilger, I. Fluorescent bacterial magnetic nanoparticles as bimodal contrast agents. Invest. Radiol. 2007, 42, 235–241. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Harrison, W.J.; Harder, J.; Wen, X.X.; Gelovani, J.G.; Qiao, A.; Li, C. Long-circulating near-infrared fluorescence core-cross-linked polymeric micelles: Synthesis, Characterization, and dual nuclear/optical imaging. Biomacromolecules 2007, 8, 3422–3428. [Google Scholar]
- Yang, Z.; Leon, J.; Martin, M.; Harder, J.W.; Zhang, R.; Liang, D.; Lu, W.; Tian, M.; Gelovani, J.G.; Qiao, A.; Li, C. Pharmacokinetics and bioredistribution of near-infrared fluorescent polymeric nanoparticles. Nanotechnology 2009, 20, 165101. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Controlled Release 2001, 73, 137–172. [Google Scholar]
- Marradi, M.; Martín-Lomas, M.; Penadés, S. Glyconanoparticles polyvalent tools to study carbohydrate-based interactions. Adv. Carbohydr. Chem. Biochem. 2010, 64, 211–290. [Google Scholar] [CrossRef]
- EI-Boubbou, K.; Huang, X. Glyco-nanomaterials: translating insights from the “sugar-code” to biomedical applications. Curr. Med. Chem. 2011, 18, 2060–2078. [Google Scholar] [CrossRef]
- Gamblin, D.P.; Scanlan, E.M.; Davis, B.G. Glycoprotein synthesis: an update. Chem. Rev. 2009, 109, 131–163. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Lu, Z.; Leow, M.L.; Ma, J.; Liu, X.W. Design of a “turn-off/turn-on” biosensor: Understanding carbohydrate-lectin interactions for use in noncovalent drug delivery. J. Am. Chem. Soc. 2012, 134, 15229–15232. [Google Scholar]
- Yang, Z.; Zhu, H. Synthesis and Revaluation of New nuclides Ga-68 Labeled D-Glucose for Potential PET imaging agent. Acta Chim. Sinica 2012, 70, 1066–1072. [Google Scholar] [CrossRef]
- Yang, Z.; Xiong, C.Y.; Zhang, R.; Zhu, H.; Li, C. Synthesis and evaluation of 68Ga-labeled DOTA-2-deoxy-d-Glucosamine as a potential radiotracer in μPET imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 499–507. [Google Scholar]
- Yang, Z.; Zhu, H.; Lin, X.F. Synthesis and evaluation of 111In-labeled D-glucose as a potential SPECT imaging agent. J. Radioanal. Nucl. Chem. 2013, 295, 1371–1375. [Google Scholar]
- Habeeb, A.F.S.A. Determination of free amino groups in protein by trinitrobenzene sulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef]
- Inman, J.K.; Dintzis, H.M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemicaladsorbents. Biochemistry 1969, 8, 4047–4082. [Google Scholar]
- Sashidhar, R.B.; Capoor, A.K.; Ramana, P. Quantitation of ε-amino group using amino acidsas reference standards by trinitrobenzene sulfonic acid. J. Immunol. Meth. 1994, 167, 121–127. [Google Scholar] [CrossRef]
- Millon, S.R.; Ostrander, J.H.; Brown, J.Q.; Raheja, A.; Seewaldt, V.L.; Ramanujam, N. Uptake of 2-NBDG as a method to monitor therapy response in breast cancer cell lines. Breast Cancer Res. Treat. 2011, 126, 55–62. [Google Scholar]
- Zhang, R.; Lu, W.; Wen, X.X.; Huang, M.; Zhou, M.; Liang, D.; Li, C. Annexin V-conjugated polymeric micelles for multimodal detection of apoptosis. J. Nucl. Med. 2011, 52, 958–964. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, H.; Zhao, J.; Lin, X.; Hong, Y.; Li, C.; Yang, Z. Design, Synthesis and Evaluation of Dual-Modality Glyco-Nanoparticles for Tumor Imaging. Molecules 2013, 18, 6425-6438. https://doi.org/10.3390/molecules18066425
Zhu H, Zhao J, Lin X, Hong Y, Li C, Yang Z. Design, Synthesis and Evaluation of Dual-Modality Glyco-Nanoparticles for Tumor Imaging. Molecules. 2013; 18(6):6425-6438. https://doi.org/10.3390/molecules18066425
Chicago/Turabian StyleZhu, Hua, Jun Zhao, Xinfeng Lin, Ye Hong, Chun Li, and Zhi Yang. 2013. "Design, Synthesis and Evaluation of Dual-Modality Glyco-Nanoparticles for Tumor Imaging" Molecules 18, no. 6: 6425-6438. https://doi.org/10.3390/molecules18066425